1. Context
Hypertension is one of the most concerning diseases of our time, serving as a major risk factor for cardiovascular disease (CVD) and contributing to nearly one-fifth of deaths in 2019 (1, 2). The most recent guidelines, including the 2023 European Society of Hypertension (ESH) and the 2017 American College of Cardiology/American Heart Association (ACC/AHA) hypertension guidelines, define hypertension using varying thresholds for systolic blood pressure (SBP) and diastolic blood pressure (DBP). The ACC/AHA defines hypertension as > 130/80 mm Hg, whereas the ESH defines it as > 140/90 mm Hg (3). According to the American Diabetes Association (ADA), hypertension in patients with diabetes is defined as ≥ 140/90 mm Hg; however, for those with cardiovascular risk factors, maintaining blood pressure below 130/80 mm Hg is recommended (4).
Different types of hypertension have specific diagnostic criteria and require tailored management approaches based on their unique characteristics. Despite advances in hypertension pharmacotherapy, a significant percentage of patients continue to experience uncontrolled hypertension (UCH) or treatment-resistant hypertension (TRH). Sympathetic nerve hyperactivity, particularly in obese patients, has been identified as a major contributing factor to these conditions (5). The primary causes of UCH and TRH include non-adherence to prescribed medications and inadequate or inappropriate selection of antihypertensive therapies (6).
1.1. Etiologies of Non-adherence to the Anti-hypertensive Medications
Several factors contribute to non-adherence to antihypertensive medications. Large-scale clinical trials have demonstrated that approximately 40 - 45% of patients with hypertension do not adhere to their prescribed medication regimens (7, 8). There is no universally accepted definition of adherence to antihypertensive therapy (9). However, according to the World Health Organization (WHO), adherence encompasses more than merely taking medications as prescribed. It also includes a patient's compliance with (1) medication, (2) a healthy diet, and (3) physical activity, as recommended by their physicians (10).
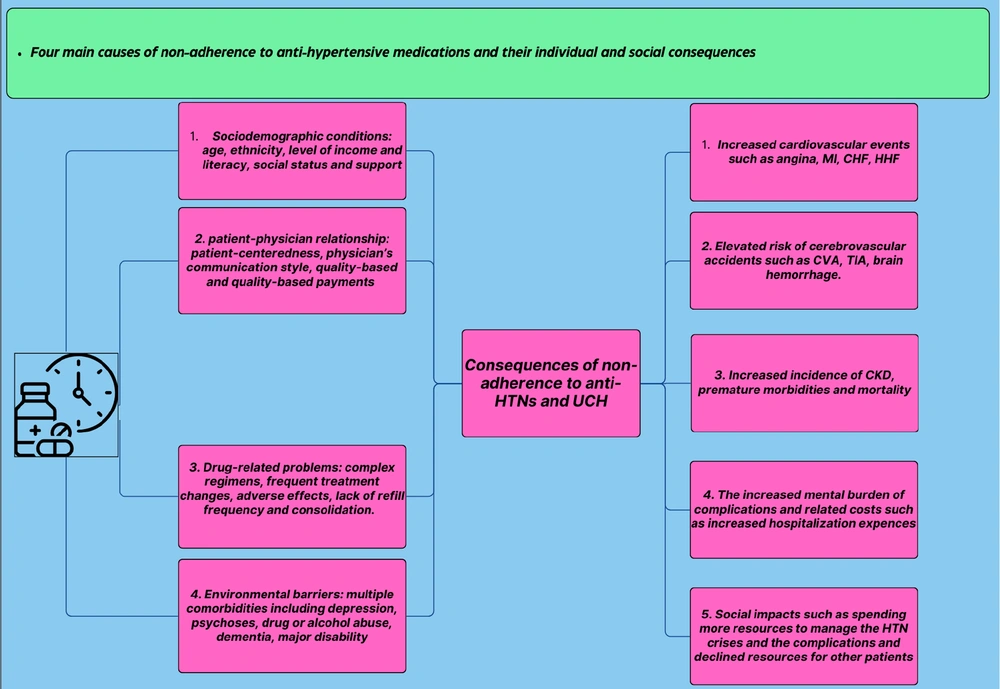
Non-adherence to antihypertensive treatment can be categorized into four major groups of contributing factors (Figure 1) (9):
(1) Sociodemographic factors: Age, ethnicity, income and literacy levels, social conditions, and the availability of social support are among the most influential factors.
(2) Healthcare system-related barriers: These include the quality of the patient-physician relationship, the payment method for healthcare services, and the extent to which decision-making processes are patient-centered.
(3) Medication-related challenges: Issues such as side effects, the complexity of medication regimens, and difficulties in timely refilling prescriptions play a significant role.
(4) Patient-related factors: A patient’s perception of their condition, knowledge about hypertension, and the presence of mental health disorders can also impact adherence (9, 11).
1.2. Social and Individual Consequences of Anti-hypertensive Non-adherence
One of the most important consequences of non-adherence to anti-hypertensive medications is a decrease in office systolic blood pressure (OSBP) and office diastolic blood pressure (ODBP), and 24-hour ambulatory blood pressure monitoring (ABPM). It is not surprising that several studies have shown that the consequences of this pathological condition and resulting UCH is an elevated risk of different types of CVD, including myocardial infarction (MI), angina, congestive heart failure (CHF), decompensated heart failure (DHF), hospitalization for heart failure (HHF), and cerebrovascular accidents (CVA) (12-15). Moreover, the evaluation of all-cause mortality (ACM), cardiovascular mortality (CVM), and survival rates was consistent among most studies, which show the statistically significant benefits of adherence to these drugs regarding the improved survival rate and declined ACM and CVM (12, 16, 17). The social perspective of this condition is also evaluated. There are several pieces of evidence that approve the hypothesis that lipid-lowering and anti-hypertensive medications and non-adherence have been associated with a significantly higher risk of hospitalization, longer inpatient days per year, higher medical and overall healthcare expenses for both individuals and societies (18-20). These effects might have been caused by their effect on the maintenance of cardiovascular health. These payments include inpatient and outpatient costs and CVD-related costs, which were considerably higher for non-adherents compared to patients with good adherence (11, 21-24).
The evaluation of renal denervation (RDN) and its effectiveness has multiple aspects. In this review, we present the evaluation by dividing it into three sections:
(1) Efficacy in treating hypertension,
(2) Efficacy in reducing the risks of major adverse cardiac events (MACE) composites, and
(3) The individual and social-psychological impacts of RDN.
1.3. Renal Denervation Background
Although the first major surgical procedure to treat hypertension was performed in 1938 by Smithwick and colleagues, interventional techniques utilizing radiofrequency emerged in 1990, and initial studies demonstrated substantial decreases in blood pressure following RDN (25). The first sham-controlled trial, the SYMPLICITY HTN-3 study, was initiated in October 2011 and published in April 2014. This trial marked a significant milestone as it aimed to address previous methodological limitations by including a sham procedure and blinding both participants and assessors. Despite high expectations, the study did not show a significant reduction in BP compared to the sham control, highlighting the complexities and challenges in establishing the efficacy of RDN. The safety of the RDN procedure was revealed in this trial. However, the pooled analysis of 535 patients showed that the difference between the RDN and control subgroups was not statistically significant regarding their 6-month office and 24-hour ABPM SBP and DBP decline.
Moreover, it has been shown that RDN has beneficial effects beyond cardiovascular diseases, including its effect on patients with diseases like chronic kidney disease (CKD) and atrial fibrillation (AF) (26). The RDN has demonstrated its benefits and safety in the management of patients with AF in several studies when applied alone or in combination with pulmonary vein isolation (PVI). Therefore, the clinical application of RDN is not limited to controlling hypertension, and many cases can benefit from this procedure (27).
Although the trial demonstrated the safety of RDN, among 535 patients identified with treatment-resistant hypertension, the difference in 6-month blood pressure decline between RDN and sham-treatment groups was not significantly different for office or ambulatory measures (28).
Recent studies have emphasized the significance of cardiovascular outcome-driven data, arguing that such data are essential to establish the clinical effectiveness of RDN beyond BP reduction alone. The need for MACE-driven trials has been highlighted to ensure the long-term benefits of RDN are accurately captured (29). In November 2023, the US Food and Drug Administration (FDA) approved the Paradise™ ultrasound catheter-based endovascular RDN system and the Symplicity Spyral™ multi-electrode RDN catheter system as new treatment options for hypertension. This approval was based on the demonstrated antihypertensive effects and the ability to improve the quality of BP reduction (30). This study aims to systematically evaluate the efficacy and safety of RDN in managing resistant hypertension, with a particular focus on long-term cardiovascular outcomes. By analyzing the most recent clinical trial data and technological advancements, we aim to provide a comprehensive overview of the potential benefits and limitations of RDN as a therapeutic intervention for patients with UCH and TRH.
1.4. Indications of Renal Denervation
This procedure involves manipulating renal sympathetic nerves to reduce BP in patients with specific BP types. An undeniable advantage of this procedure is that it is minimally invasive. It has gained attention as a potential treatment for patients who do not achieve adequate BP control with medication alone (31), given the critical role of renal sympathetic nerves in the neurogenic control of blood pressure and the pathophysiology of hypertension (32). The RDN is primarily indicated for patients with the following conditions: (A) Refractory Hypertension: Patients with an OSBP > 160 mmHg despite taking ≥ 3 antihypertensive medications, which include at least a diuretic, in their optimal doses; (B) exclusion of Secondary Hypertension: Patients must be evaluated to rule out secondary forms of hypertension; (C) exclusion of white coat hypertension: Ambulatory blood pressure monitoring should be used to exclude white coat hypertension (33, 34). The procedure has contraindications; although there is no common consensus among various guidelines, the most commonly agreed exclusion criteria include increased bleeding risk, chronic renal disease with an estimated glomerular filtration rate (eGFR) less than 45 mL/min/1.73 m², type 1 diabetes, previous renal artery intervention, anatomical abnormalities of the renal arteries, age under 18, and pregnancy (33, 35).
2. Evidence Acquisition and Results
2.1. Effects of Renal Denervation Treatment on Different Subtypes of Blood Pressure
Renal denervation has emerged as a promising therapeutic strategy for managing resistant hypertension through the modulation of renal sympathetic nerve activity. This section reviews the impact of RDN on various BP measurements, providing insights into the comprehensive benefits and limitations of this treatment across different settings, including 24-hour ambulatory, home, office, daytime, and nighttime systolic and diastolic blood pressure (Table 1).
| Trials | Year | Device | Energy Source | Number | FU, (mo) | |
|---|---|---|---|---|---|---|
| RD | Sham | |||||
| Symplicity HTN-3 | 2014 | Symplicity Flex Catheter | Radiofrequency | 364 | 171 | 6 |
| Symplicity FLEX | 2015 | Symplicity Flex Catheter | Radiofrequency | 35 | 36 | 6 |
| Reset | 2016 | Symplicity Flex Catheter | Radiofrequency | 36 | 33 | 6 |
| Radiance-HTN solo | 2018 | Paradise | Ultrasound | 74 | 72 | 2 |
| SPYRAL HTN-off MED pivotal | 2020 | Symplicity Spyral and G3 | Radiofrequency | 166 | 165 | 3 |
| Reduce HTN: Reinforce | 2020 | Vessix System | Radiofrequency | 34 | 17 | 2 |
| Radiance-HTN TRIO | 2021 | Paradise | Ultrasound | 69 | 67 | 2 |
| Require | 2021 | Paradise | Ultrasound | 69 | 67 | 3 |
| Heradien et al. | 2022 | Symplicity Flex and Spyral | Radiofrequency | 42 | 38 | 6 |
| Pathak et al. | 2023 | Peregrine | Alcohol | 50 | 56 | 2 |
| RADIANCE II | 2023 | Paradise | Ultrasound | 150 | 74 | 2 |
| SPYRAL HTN-ON MED (expansion) | 2023 | Symplicity Spyral and G3 | Radiofrequency | 206 | 131 | 6 |
| SPYRAL HTN-ON MED (long-term) | 2022 | Symplicity Spyral and G3 | Radiofrequency | 36 | 42 | 3 |
| Simplicity HTN-3 (long-term) | 2022 | Symplicity Flex Catheter | Radiofrequency | 364 | 171 | 6 |
| Target BPI | 2024 | Peregrine | Alcohol | 145 | 146 | 6 |
| SMART | 2024 | Catheter | Radiofrequency | 109 | 110 | 6 |
Included Clinical Trials and Their Primary Features
2.1.1. 24-Hour Ambulatory Blood Pressure
The most recent systematic review and meta-analysis conducted by Vukadinović et al. (2024) examining the effects of RDN have demonstrated significant reductions in 24-hour systolic ABPM. The meta-analysis, which included ten eligible trials, showed that the denervation process substantially reduced 24-hour ABPM by 4.4 mmHg (P < 0.00001). This sustained reduction over 24 hours suggests a continuous antihypertensive effect, highlighting the potential of RDN in managing overall BP levels throughout the day and night (36). Further supporting these findings, Ogoyama et al. (2024) conducted a comprehensive systematic review and meta-analysis that included 12 sham-controlled trials with 2,222 patients. This study showed a significant reduction in 24-hour ABPM of SBP by -2.81 mmHg (95%CI: -4.09 to -1.53, P < 0.001) compared to the control group. This reduction was consistent across subgroups, including different RDN devices and patient populations with or without concurrent antihypertensive medication use (37).
In a systematic review and meta-analysis conducted by Sobreira et al., ten studies encompassing a total population of 1,066 patients were analyzed, revealing that the intervention group exhibited a mean decrease in 24-hour systolic BP of nearly 5 mmHg. This finding underscores the efficacy of RDN in achieving sustained BP control over a full day beyond the reductions observed in office measurements. The relatively low heterogeneity (I² = 34%) suggests a consistent effect across the included studies, bolstering the robustness of this result (38).
Similarly, 24-hour DBP shows notable reductions following RDN. The study by Vukadinović et al. (2024) reported an average decrease of about 2.5 mmHg in 24-hour DBP in patients undergoing RDN compared to sham procedures, showing a statistically significant difference. These findings support the efficacy of RDN in providing a stable reduction in diastolic pressures, contributing to improved cardiovascular outcomes (36). Ogoyama et al. (2024) also found significant reductions in 24-hour ambulatory DBP, with an average decrease of almost 1.50 mmHg compared to the sham procedure, which was significantly more than the control group. This study further reinforces the potential of RDN to offer continuous BP control throughout the day and night, thereby reducing overall cardiovascular risk (37). Similarly, 24-hour diastolic BP showed a significant reduction in the RDN group, with a mean decrease of 2.3 mmHg (95% CI −4.19 to −0.52; P = 0.012; I² = 59%). Although the heterogeneity for diastolic BP was higher than for systolic BP (I² = 59%), the results remained statistically significant, indicating a notable impact of RDN on DBP control. These reductions in 24-hour ABPM parameters highlight the potential of RDN to offer continuous cardiovascular protection by maintaining lower BP levels throughout the day and night, which is critical for reducing the risk of hypertensive complications and improving long-term outcomes in patients with resistance (38).
2.1.2. Systolic and Diastolic Office Blood Pressure
Although office blood pressure may be affected by the white coat effect (hypertension), it is still measured as part of the diagnostic process for this disease. The effect of RDN on office blood pressure (OBP) has been investigated in several trials (37). Most studies have categorized the measured OBP into two subgroups: Systolic OBP (SOBP) and diastolic OBP (DOBP). The first trials investigating the effectiveness of SOBP were Symplicity HTN-I and II, conducted in 2009 and 2010, respectively (39, 40). Both trials demonstrated significant differences between the RDN and control groups in short- and long-term (36-month) evaluations (39, 41). These long-term beneficial effects were among the first promising results, encouraging researchers to focus more on this alternative treatment for one of the most serious health concerns.
2.2. Renal Denervation Effect on Declining Major Adverse Cardiovascular Events
It has been shown in experimental and observational studies that multiple CVD composites have declined in response to RDN (42). For example, in MI, one of the most important causes of ACM and CVM, the preclinical and clinical consequences of RDN were all toward improved outcomes. Specifically, catheter renal ablation has improved reperfusion, reduced infarct volume, and decreased MI-induced inflammation and oxidative stress (43). Other mechanisms found in various studies that result in the reduced adverse effects of RDN on MI patients include improved left ventricular (LV) remodeling, with less prominent myocardial changes such as fibrosis and hypertrophic cardiomyopathy (HCM), as well as decreased inflammation infiltration (42). Moreover, in the context of heart failure (HF), CHF, DHF, and hospitalization for HHF, important components of CVD, RDN has been associated with decreased norepinephrine secretion and improved signs and symptoms of HF. As a result, CHF, DHF, and HHF are all reduced, leading to improved CVD outcomes and a reduction in ACM and CVM (44-46).
2.3. Renal Denervation Effects on the Quality of Life, Mental Health, and Psychological Disorders
Considering the fact that one of the most prominent causes of UCH is the misperception caused by a loss of decision compliance and mental disorders such as depression and anxiety (47), this treatment can result in specific benefits for patients with these types of disorders (48). With a single procedure that can be effective for a long time, the negative effects of non-adherence are mitigated by this treatment. Therefore, RDN should be considered a potentially lifesaving alternative to antihypertensive medications for a significant percentage of people who do not use prescribed medications due to mental disorders. To support this hypothesis, several pieces of evidence suggest that this treatment can improve the quality of life (QOL) of UCH patients and also reduce the incidence and prevalence of health-related depression and anxiety (49). Lambert et al. evaluated these patients' post-renal denervation QOL at several intervals. They found that the SF-36 score, which represents mental health conditions and symptoms of the BID, was significantly improved about 90 days after the procedure (49). Interestingly, this effect has been shown to be different from the BP-lowering effects of these medications. A hypothesis for this independent effect is that there has been a long-term decline in concern about UCH. However, considering the observational nature of the study design, it is insufficient to conclude causality. On the other hand, some studies have reported a decline in QOL in UCH and TRH (49-52). Therefore, it seems reasonable that controlling a condition associated with decreased QOL can improve mental health and life satisfaction.
Another study performed by Lenski et al. evaluated anxiety, depression, and QOL in 119 patients with TRH and found that all psychological factors evaluated were significantly improved after the procedure. The patients’ depression, anxiety, headache, and QOL had a higher average after RDN in this study (53). They attributed at least part of this effect to the common regulatory pathway between BP regulation and stress and anxiety. The sympathetic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis manage stress reactions and BP regulation (54-56). This effect on a larger scale could be seen in societies if this treatment becomes accessible to all patients eligible for this treatment from different backgrounds (57). Examining the cost-effectiveness of modern catheter-based RDN for TRH patients showed significantly lower average costs, cardiovascular disease-related morbidity, and mortality (58).
3. Conclusions
Evaluating available RCTs, systematic reviews, meta-analyses, and observational studies has shown that RDN is an effective treatment for reducing office and 24-hour SBP and DBP. Moreover, this treatment can decrease the incidence rate of all cardiovascular-related events, such as ACM, CVM, and CVA. Although fewer studies have been evaluated, psychological and psychiatric assessments of patients indicate that this treatment can reduce the burden of depression and anxiety on both individual and societal scales. Therefore, this treatment can significantly improve the management of hypertension and the resulting complications of this common disease, such as CVD and CVA. It also has the potential to improve mental health disorders such as depression, anxiety, and quality of life. The average cost of treating hypertension and its complications can also be positively impacted by the approval and use of RDN on a large social scale.
3.1. Strength and Limitations
The main strength of this study lies in evaluating a wide range of factors associated with the effects of RDN, including both physical and mental disorders, in a relatively large pool of studies conducted on patients with various baseline characteristics. On the other hand, the inclusion of various types of studies with different subjects and methodologies has resulted in a heterogeneity of information, which could potentially introduce bias.