1. Background
Cadmium (Cd) is a highly toxic element with no positive physiological function for plants and humans (1). Cd is accumulated in different plant organs (2) and can be consumed if these plants are used as food. Excessive intake of Cd leads to serious medical problems such as itai-itai disease, lung cancer, gastrointestinal cancer, kidney damage, and liver disease (3). Therefore, it is necessary to either clean Cd from soil or control its uptake by plants to enhance the environmental quality and human health (4). To achieve these aims, different methods such as soil washing, excavation, and land filling have been developed (5). However, due to high costs and difficulties in removing toxic metals from soil and water by conventional methods, research is presently focused on low-cost as well as easy and safe technologies (6).
Cd and Zinc (Zn) have similar geochemical and environmental properties, which causes an antagonistic interaction between them during plant uptake, transport from roots to the aerial parts, or accumulation in edible parts of plants (7-9). On the other hand, it has well known that Zn is not only an essential micronutrient for humans and animals (10), however, it also supports growth and yields the quality of plants (11).
It has been reported that some agricultural lands in Iran are contaminated with Cd due to industrial activities, high application of low-quality phosphate-fertilizers containing Cd as impurities, and geological properties (12). Additionally, plant available Zn in soils of central part of Iran such as Arak is low (13), and use efficiency of Zn fertilizers in soils is limited due to natural soil physic-chemical properties (14). Application of organic matter, such as cow manure, is an important strategy to enhancing micronutrient bioavailability and improving use efficiency of different kinds of micronutrient fertilizers in low organic matter soils such as soils of the central part of Iran.
Canola (Okapi Cv.) is cultivated in central parts of Iran periodicity with wheat and is consequently recommended by the Markazi Agricultural and Natural Resource Research Centre to cultivate in Markazi province due to its adaptation to climatic conditions of this area. However, canola is a high sensitive plant to Zn deficiency.
Tire rubber is a good source of Zn, it contains of approximately 1% - 2% of Zn (15), and could be a profit fertilizer especially in an area with low soil Zn bioavailability such as Iran (16). In a study by Taheri et al., the amount of Zn increased significantly when calcareous Zn-deficient soil was amended with tire rubber ash (16). On the other hand, Baghaie showed that applying 40 t ha-1 tire rubber ash enriched municipal waste compost significantly decrease the spinach Cd concentration (17). According to Khoshgoftarmanesh et al. (18), applying tire rubber ash is an important way to reduce plant Cd concentration due to the completive effect of Cd and Zn.
2. Objectives
This study was done to investigate the influence of tire rubber ash enriched cow manure on Cd bioavailability in soil and canola Cd concentration in a Zn deficient soil that is polluted with Cd.
3. Methods
3.1. Study Design
A factorial experiment with a randomized complete block design in three replications was done in the greenhouse. The factors were zero (C0), 10 (C10), and 20 (C20) t ha-1 cow manure, 0 (T0) and 200 (T200) kg ha-1 tire rubber ash, and zero (Cd0), five (Cd5), 10 (Cd10), and 15 (Cd15) mg Cd (kg soil)-1.
3.2. Soil Origin and Physicochemical Properties
The soil used for this experiment was Typic Haplocambid (19) collected from the top 30 cm soil layer of a field at Pakal village, located 30 km west of Arak. The soil texture was a silty clay loam based on the hydrometer method (20). The pH was 7.1 and the electrical conductivity (EC) was 0.84 dSm-1. The soil organic C was 0.17%, based on the Walkley and Black methods (21). The cation exchangeable capacity (CEC) was 11 Cmol(+) (kg soil)-1 as determined by the Rhoads (22). The DPTA extractable Cd and Zn was analyzed using the Lindsay method (23). The amount of DTPA-extractable Zn was 0.12 mg (kg soil)-1. The DTPA-extractable Cd was not detectable by atomic absorption spectroscopy (AAS).
3.3. Preparation of Tire Rubber Ash
Experimental tire rubber was prepared from the rubber industry of Yazd tire in Iran and then ashed in a furnace at 550°C for 12 hours. The total concentration of Zn, Fe, Cd, and Pb of the tire rubber ash were 10840, 83.4, 7.96, and 0.94 mg (kg DW)-1, respectively, according to energy dispersive X-ray fluorescence spectrometry (XRF) on a Spectra X-Lab 2000 instrument (SPECTRO Analytical Instruments GmbH, Kleve, Germany).
3.4. Cow Manure Chemical Analysis
The total concentration of Zn, Cd, and Pb in the cow manure were 98.32, 0.61, and 0.73 mg (kg DW)-1, respectively. The organic C of cow manure was 18.12% and the CEC was 9.33 Cmol(+) (kg soil)-1. The pH of cow manure was 7.22. The total N of samples was 1.3%, according to the Kjeldahl method (24).
3.5. Soil Preparation
To have tire rubber ash enriched cow manure, different levels of cow manure were enriched by different levels of tire rubber ash and incubated for three months in a room with a temperature of 25°C. Then, four levels of Cd in the form of CdNO3 were sprayed in the soil samples and incubated in a room for three months. After incubation time, the tire rubber ash enriched cow manure were added to the Cd treated soils and mixed roughly to assure its homogeneous distribution.
3.6. Growth Conditions and Mineral Nutrient Analyses
Canola seedling were first surface sterilized in 15% H2O2 thoroughly washed in distilled water, and pre-germinated on moistened filter paper. After that, two canola seedlings were planted into each pot with five kg soil. At harvest, aboveground parts and the roots of plants were washed and dried at 75°C for 72 hours and weighted.
For chemical analyses, the milled samples were incinerated at 550°C for six h, dissolved in one ml of 13 M HNO3 and heated in 220°C for one minute (25). The concentrations of Cd and Zn were determined by AAS (800 model). The accuracies of Cd and Zn analyses were controlled by analyzing certified standard materials. Soil pH, CEC, total N content, and DTPA-extractable Cd and Zn were measured at the end of the experiment, using the above-described analytical procedures.
3.7. Plant Growth Factor Index
To determine the ability of canola to grow in different research treatments, the plant growth factor index (GI) was calculated as below (26):
GI (%) = (DW of treated plants) / (DW of control plants) × 100 (1)
Where DW is the dry weight of canola aboveground biomass.
4. Results
4.1. Soil Chemical Properties
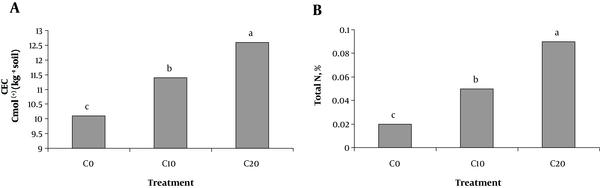
Soil pH was not affected by any of the treatments (data not shown). Cow manure addition significantly increased CEC and total soil N, irrespectively from the tire rubber ash and Cd concentration (Figure 1A and B). The total N content in soils treated with cow manure was significantly (P < 0.05( higher than in those that did not (Figure 1B).
The concentration of soil DTPA-extractable Cd was significantly higher in the treatments without tire rubber ash enriched cow manure than with it (Table 1). The highest concentration of plant available Cd in soil (6.3 mg kg DM-1) was observed in soils treated with the highest level of Cd but amended with neither tire rubber ash nor with cow manure (Table 1). The concentration of DTPA-extractable Zn in soils was significantly higher in the treatments with tire rubber ash enriched cow manure than without it. Increasing the Cd concentration of soil significantly reduced soil DTPA-extractable Zn concentration (Table 1).
| Treatment | T200Cd15 | T200Cd10 | T200Cd5 | T200Cd0 | T0Cd15 | T0Cd10 | T0Cd5 | T0Cd0 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Zn | Cd | Zn | Cd | Zn | Cd | Zn | Cd | Zn | Cd | Zn | Cd | Zn | Cd | Zn | |
| C0 | 5.6b | 0.06lm | 3.7de | 0.12jk | 2.6hi | 0.19gh | ND | 0.28de | 6.3a | 0.01n | 4.5c | 0.04mn | 3.4ef | 0.08klm | ND | 0.12jk |
| C10 | 4.9c | 0.10jkl | 3.1fg | 0.18ghi | 2.1j | 0.25ef | ND | 0.38b | 5.6b | 0.07lm | 3.7de | 0.09kl | 2.7gh | 0.14ij | ND | 0.21fgh |
| C20 | 3.9d | 0.14ij | 1.5k | 0.22fg | 0.2m | 0.31cd | ND | 0.49a | 4.6c | 0.12jk | 2.2ij | 0.17hi | 0.9l | 0.24ef | ND | 0.35bc |
Effects of Cow Manure Application Rate (C), Tire Rubber Ash Addition (T), and Soil Cadmium Concentration (Cd) on Available Cd and Zn-DTPA Extractable Concentration in Soil (mg (kg soil)-1)a
4.2. Plants Biomass
The aboveground biomass of canola was significantly affected by all of the treatments (Table 2). The highest biomass (5.80 g pot-1) was observed for plants grown in soil without addition of Cd (0 Cd level), however, was amended with cow manure and tire rubber ash (Table 2). The lowest aboveground biomass (3.50 g pot-1) was recorded for plants grown in soils with addition of 15 mg Cd (kg soil)-1, however, without cow manure or tire rubber ash (Table 2).
| Treatment | T200Cd15 | T200Cd10 | T200Cd5 | T200Cd0 | T0Cd15 | T0Cd10 | T0Cd5 | T0Cd0 |
|---|---|---|---|---|---|---|---|---|
| C0 | 3.9n | 4.4jk | 4.7gh | 5.2de | 3.5o | 4.1m | 4.3kl | 4.6hi |
| C10 | 4.5ij | 4.7gh | 5.1e | 5.4c | 4.1m | 4.3kl | 4.8fg | 5.1e |
| C20 | 5.1e | 5.3c | 5.6b | 5.8a | 4.9f | 5.1e | 5.3c | 5.5bc |
Effects of Cow Manure Application Rate (C), Tire Rubber Ash Addition (T), and Soil Cadmium Concentration (Cd) on the Aboveground Biomass of Plants g (pot)-1 a
4.3. Plant Growth Index
The highest value of GI was observed in plants grown on non-Cd treated soil with the highest ratio of cow manure and tire rubber ash (Table 3). The GI of canola plants was significantly reduced when the level of Cd in soil increased. Application of cow manure and tire rubber ash to the soil reduced the negative effect of Cd on plant GI (Table 3). The highest levels of cow manure and tire rubber ash in soils with 15 mg Cd (kg)-1 increased the GI by 0.25 units compared to GI of canola plants grown in soils with the same concentration of Cd but without cow manure and tire rubber ash (Table 3).
| Treatment | Cd0 | Cd5 | Cd10 | Cd15 | ||||
|---|---|---|---|---|---|---|---|---|
| T0 | T200 | T0 | T200 | T0 | T200 | T0 | T200 | |
| C0 | 1.00gh | 1.10de | 0.91kl | 1.00gh | 0.87m | 0.94jk | 0.76o | 0.84n |
| C10 | 1.08e | 1.15c | 1.02fg | 1.08e | 0.91l | 1.00gh | 0.87m | 0.95ij |
| C20 | 1.18b | 1.34a | 1.14c | 1.19b | 1.08e | 1.12cd | 1.04f | 1.09e |
Effects of Cow Manure Application Rate (C), Tire Rubber Ash Addition (T), and Soil Cadmium Concentration (Cd) on Plant Growth Index (GI)a
4.4. Accumulation of Cd and Zn in Canola Plants
Grain Cd concentration significantly (P < 0.05) decreased with application of cow manure and tire rubber ash (Table 4), whereas the Zn concentration significantly (P < 0.05) increased (Table 4). Regardless of non-Cd polluted soil, the lowest grain Cd concentration (0.9 mg (kg DW)-1) was recorded for plants grown at the lowest application rate of Cd (5 mg (kg soil)-1) with receiving the highest amount of cow manure and tire rubber ash. The highest grain Zn concentration (11.6 mg (kg DW-1) was observed for plants grown in the absence of Cd but in the presence of cow manure and tire rubber ash (Table 4).
Regardless of non-Cd polluted soil, the lowest Cd concentration in plants roots (7.2 mg (kg DW-1)) was observed when the soils amended with the highest amount of cow manure and tire rubber ash, while the root Zn concentration was increased (Table 4).
The Cd concentration in shoots and grains were significantly correlated with the application rate of cow manure, tire rubber ash, and Cd, according to the models below:
Grain Cd concentration = 6.03 - 0.20 Vi - 0.006 Tj + 0.16 Cdk, R2 = 0.83
Shoot Cd concentration = 9.32 - 0.14 Vi - 0.006 Tj + 0.31 Cdk, R2 = 0.94
where, Vi is cow manure levels, Tj is tire rubber ash levels, and Cdj or Cdk is soil cadmium concentration.
| Treatment | T200Cd15 | T200Cd10 | T200Cd5 | T200Cd0 | T0Cd15 | T0Cd10 | T0Cd5 | T0Cd0 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Zn | Cd | Zn | Cd | Zn | Cd | Zn | Cd | Zn | Cd | Zn | Cd | Zn | Cd | Zn | |
| Grain Zn and Cd concentration (mg (kg DWl)-1) | ||||||||||||||||
| C0 | 9.4c | 5.1op | 5.3f | 5.8mn | 2.7jk | 6.4klm | ND | 7.2ij | 12.2a | 3.6q | 6.1e | 4.5p | 3.1i | 5.4no | ND | 6.8jk |
| C10 | 7.8d | 6.9jk | 3.7h | 7.8hi | 1.9l | 8.9fg | ND | 9.6de | 10.1b | 5.9lmn | 4.8g | 6.5kl | 2.4k | 3.7q | ND | 8.6fg |
| C20 | 5.1fg | 8.8fg | 2.9ij | 9.2def | 0.9m | 10.7b | ND | 11.6a | 6.3e | 8.3gh | 3.9h | 9.1ef | 1.6l | 9.8cd | ND | 10.4bc |
| Shoot Zn and Cd concentration (mg (kg DWl)-1) | ||||||||||||||||
| C0 | 20.1b | 6.8m | 14.1f | 7.9kl | 10.1k | 8.6ij | ND | 9.8fg | 24.6a | 4.3o | 15.6d | 6.5m | 12.2h | 7.4l | ND | 9.2h |
| C10 | 15.6d | 7.9kl | 11.5i | 8.2jk | 9.1l | 10.3ef | ND | 11.6c | 18.8c | 5.6n | 13.5g | 8.2jk | 10.0k | 9.1hi | ND | 10.8de |
| C20 | 11.1j | 7.8kl | 8.5m | 10.3ef | 7.2o | 12.2b | ND | 13.9a | 14.8e | 6.4m | 10.8j | 9.3gh | 8.1n | 11.1cd | ND | 12.6t |
| Root Zn and Cd concentration (mg (kg DWl)-1) | ||||||||||||||||
| C0 | 52.1b | 10.0n | 40.5f | 12.1ijk | 30.0jk | 13.8efg | ND | 14.9cd | 56.4a | 7.1p | 45.6d | 9.2o | 34.8h | 10.8m | ND | 11.6kl |
| C10 | 42.6e | 11.8jk | 33.2i | 13.2gh | 25.7m | 14.4de | ND | 16.3b | 47.3c | 9.4no | 40.6f | 12.3ij | 29.1kl | 13.6fg | ND | 15.2c |
| C20 | 38.2g | 12.7hi | 28.3l | 14.2ef | 19.7o | 16.2b | ND | 17.9a | 40.6f | 11.1lm | 31.3j | 13.3gh | 23.9n | 15.1d | ND | 16.8b |
Effects of Cow Manure Application Rate (C), Tire Rubber Ash Addition (T), and Soil Cadmium Concentration (Cd) on Grain, Shoot and Root Zn and Cd Concentration (mg (kg DW)-1)a
5. Discussion
5.1. Cd and Zn Availability in Soil
Addition of Cd to soils without receiving cow manure and tire rubber ash reduced the DTPA-extractable Zn from 0.12 to 0.01 mg (kg soil-1), which suggest that Zn and Cd may compete with each other for sites available for sorption on soil colloids (17). Cd and Pb have similar chemical and geological properties (27), which may predispose them to behave as competitors for sorption sites (soil phase) or uptake (plant phase). In this way, other authors also have reported that in Cd contaminated soils, the bioavailability of Zn is low (28).
Application of tire rubber ash enriched cow manure increased the DTPA-extractable Zn concentration (up to 0.49 mg (kg soil-1), Table 1). Elevated root and microbial exudation in response to addition of cow manure to soil may be a reason of higher DTPA-extractable Zn concentrations in soil (25). In addition, the role of organic amendments such as cow manure on decresing Cd concentration cannot be ignored. Baghaie reported that applying manucipal waste compost increase soil organic matter that may increase the soil sorption site and thereby deecrease soil Cd concentration (20). The research of Tabarteh et el. (2017) confirm this matter clearly (29).
5.2. Growth, Cd and Zn Concentration, GI of Canola Plants
Application of tire rubber ash enriched cow manure increased plants biomass (Table 1). It could be due to plant-related processes, e.g. improved N and C nutrition in the presence of the cow manure, and/or to microorganisms-related processes, e.g. mobilization of nutrients by root-associated microbes. In contrast, plants biomass was significantly reduced when concentration of Cd increased (Table 1), which stays in agreement with previously published research. For example, it has been reported that high concentration of Cd prevents the normal uptake, translocation and usage of mineral nutrients, and leads to reduction in plant growth and development (30, 31).
Addition of tire rubber ash enriched cow manure to Cd polluted soil (15 mg (kg soil)-1) and reduced Cd concentration of canola significantly (Table 4), which may be due to enhanced uptake of Zn. However, this Cd concentration is still greater than the standard value for plants (32).
A higher concentration of Zn in grains of plants grown in the presence of tire rubber ash enriched cow manure may imply synergistic effect of these two compounds on uptake of Zn. We assume that as tire rubber ash is served as a direct source of Zn, cow manure probably facilitated its uptake by changes in soil structure, enhanced activity of microbes mobilizing Zn from tire rubber ash, or other processes that are not fully explained yet. Aghili et al. reported that application of organic amendments to Zn deficient soil can increase mycorrhizal activity and Zn uptake by plant. In addition, it has been reported that N content in organic amendment can increase microbial activity and thereby increase the concentration of some components such as organic acids that increase Zn mobilization in soil (25). Overall, N mediated effects on Zn uptake (33) and translocation in plants might be caused by the following mechanisms: (i) Stimulation of the release of Zn phyto-siderophores to the rhizosphere, (ii) stimulation of the synthesis of Zn transporter proteins expressed in the root epidermis and root hairs, and/or (iii) facilitation of intra-plant Zn re-mobilization from vegetative plant tissues to grains via stimulation of the synthesis of nitrogenous compounds. Enhanced plant growth and reduction of plant Cd concentration when canola grew in the presence of tire rubber ash enriched cow manure may also explain differences in GI value in different treatments.
5.3. Conclusions
The results of our study demonstrated that addition of tire rubber ash enriched cow manure to calcareous, Cd polluted, and Zn deficient soil is a viable strategy to bio-fortify canola with Zn and also reduces Cd concentration in canola grain. These findings are relevant to practice and thus, may help to implement locally available products (tire rubber ash and cow manure) into agricultural production. This in turn may be an important alternative to minimize Zn deficiency and Cd toxicity in the diet of people on the local scale. However, this research needs to be tested in the field location. On the other hand, the soil texture, the type of soil pollution, and plant physiology have important roles on plant heavy metal concentration. Therefore, the role of sorption properties of organic addition in decreasing Cd concentration can not be ignored.