1. Background
Cadmium is one of the heaviest metals in the natural environment that is used by many factories. Increasing the concentration of cadmium in liver cells can increase the damage of DNA in these cells (1). Also, cadmium can increase caspases-3, 8, and 9, and increase the level of mRNA for caspases-3 and 8, as well as Bax, which in turn increases the amount of apoptosis in cells. On the other hand, cyclin-D is one of the most frequent genes in tumors (2).
Based on previous studies, it has been shown that increasing the amount of mir202 reduces the expression of the cyclin-D gene, and stops cell division and induction of apoptosis (3). Micronutrient selenium is a part of antioxidant cysto-proteins. Selenium with its own effect reduces the production of free radicals and restores hydroperoxide mediums (4). In fact, selenium, by boosting the antioxidant defense system, accelerates recovery and reduces inflammation (5).
It has also been shown that in selenium-receiving rats, the anti-oxidant glutathione peroxidase capacity increased as compared to the control group; and in the selenium-receiving group with training, as compared to rats in the training group, a significant decrease in malondialdehyde and a significant increase in the total antioxidant capacity and glutathione were found (4).
Sports activities with different intensities have been reported to have different effects on the apoptotic process. For example, in one study, it was found that high intensity exercise could increase the gene expression of PGC-1α, which in turn increased the biogenesis of mitochondria, and since mitochondria plays an important role in apoptosis, it can decrease cellular degradation (6). A study conducted with an over-training protocol showed that caspase-3 liver tissue was less in the down hill training group compared to the control group, and although over-training protocol reduced stressors, it had no correlation with apoptosis in mice. Contrary to these findings, it has been reported that in rats with cardiac infarction, six weeks of high intensity interval training is more effective than low intensity interval training in increasing caspase-3 (which includes caspases for the production of caspase-3) (7).
2. Objectives
Regarding the contradictions in the findings of the reported studies and the effects of selenium on the reduction of the production of free radicals and inflammation, as well as the strengthening effect of selenium on the antioxidant defense system, the present study sought to investigate the interactive effects of interval training with selenium consumption on the gene expression of caspase-3 and cyclin-D in the liver tissue of rats exposed to cadmium.
3. Methods
The present research was an experimental study. In this study, 30 male Sprague Dawley rats with an approximate age of eight weeks were purchased from the Islamic Azad University of Marvdasht and transferred to the Sport Physiology Laboratory of this university. Animals were kept in a standard situation (temperature of 23 ± 2°C; light/dark cycle of 12:12, and humidity of 45% to 55%). During the research period, standard food plate and water was provided freely. After seven days, rats were divided to six groups of five rats including: (1) cadmium, (2) selenium with cadmium, (3) interval training with cadmium, (4) interval training with selenium and cadmium, (5) sham, and (6) control. For eight weeks, rats in groups one to four received 2 mg/kg of cadmium peritoneally per day (8). It should be noted that during the eight weeks, the control group did not receive selenium, training, and cadmium, and the sham group received normal saline peritoneally. Rats in groups two and four consumed 0.23 mg/kg of selenium daily (9). As in a previous study, the safety dose of cadmium and selenium for rats was reported (8, 9); in the present study, 2 mg/kg of cadmium consumption and 0.23 mg/kg of selenium consumption was prescribed. Also, groups three and four performed selected interval trainings for eight weeks; three sessions per week.
3.1. Training Protocol
In order to perform interval training, the maximum oxygen consumption (VO2max) of all rats was measured by Bedford et al.’s standard test (10). The interval training included a combination of high intensity and low intensity interval repeats. High intensity interval repeat included two minutes with maximum intensity of 80% in the first week, 90% maximum in the second week, 100% maximum in the third week and 110% maximum speed from the beginning of the fourth week until the end of the training, and low intensity interval repeat (recoveryinterval) included two minutes with a maximum intensity of 50%. After performing the last high intensity interval repeat, the rats were allowed to cool down for five minutes at 60% to 60% intensity. The number of high-intensity interval repeats was determined according to the weekly training period of rats so that in the first week, two repeats of high-intensity interval; in the second week, four repeats of high intensity interval; in the third week, six repeats of high intensity interval; and since the beginning of the fourth week, eight repeats of high intensity interval were included.
3.2. RNA Extraction, cDNA Synthesis and Real-Time-Polymerase Chain Reaction
At the end of the study, 48 hours after the last training session, selenium injection and cadmium consumption, the rats were anesthetized by peritoneal injection of ketamine and xylazine, and then they were sacrificed and liver tissues were used to measure the caspase-3 and cyclin-D. Total RNA was extracted from 30 mg of tissues. Two step real time PCR was performed in two separate reactions to evaluate the gene expression. All reagents, including probes and primers, were obtained from Applied Biosystems, USA. TaqMan probe (known as fluorogenic 5’ nuclease) was chosen to perform multiplex PCR. Primers were designed by the same company for specific targets: caspase-3: Rn00563902; catalogue no: 4331182, and cyclin-D: Rn014844001; catalogue no: 4351372,47 amplifies 98 bp segment of caspase-3 from the whole mRNA length of 2474 bp, and 78 bp segment of cyclin-D from the whole mRNA length of 1482 bp, respectively. BETA ACTIN and GAPDH were used as reference genes. All amplification experiments were done in three biological replicates. Amplification program included 15 minutes at 48°C (reverse transcriptase), 10 minutes at 95°C activation of ampliTaq gold DNA polymerase, denaturation at 95°C for 15 seconds and annealing at 60°C for one minute. Denaturation and annealing steps were performed for 40 cycles. Step One Plus real time PCR machine, TaqMan Fast Advanced Master Mix, and assays were purchased from Applied Biosystems, USA. The assay used TaqMan1-caspase-3: Rn00563902; catalogue no: 4331182, and cyclin-D: Rn014844001;catalogue no: 4351372. Data was analyzed according to the comparative Ct (2-ΔΔCt) method, where amplification of the target and of the reference genes were measured in the samples and reference. Measurements were normalized using the Gen Ex software. Data Assist V3 software from Applied Biosystems, USA, was used to calculate the RNA fold changes. The sequences of the primers were as below:
- Caspas-3:
Forward: ATGGGAGCAAGTCAGTGGAC -3'-5'
Reverse: GTACCAGAGCGAGATGACA-3'-5'
- Cyclin-D:
Forward: AGGCCGGGTGAGACCAGGCC-3'-5'
Reverse: TCCTGAACCTTGTGCAGCAT-3'-5'
3.3. Statistical Analysis
SPSS software, Kolmogorov-Smirnov, Two-way ANOVA, and One-way ANOVA with Tukey’s post hoc tests were used for analyzing the research findings (P ≤ 0.05).
4. Results
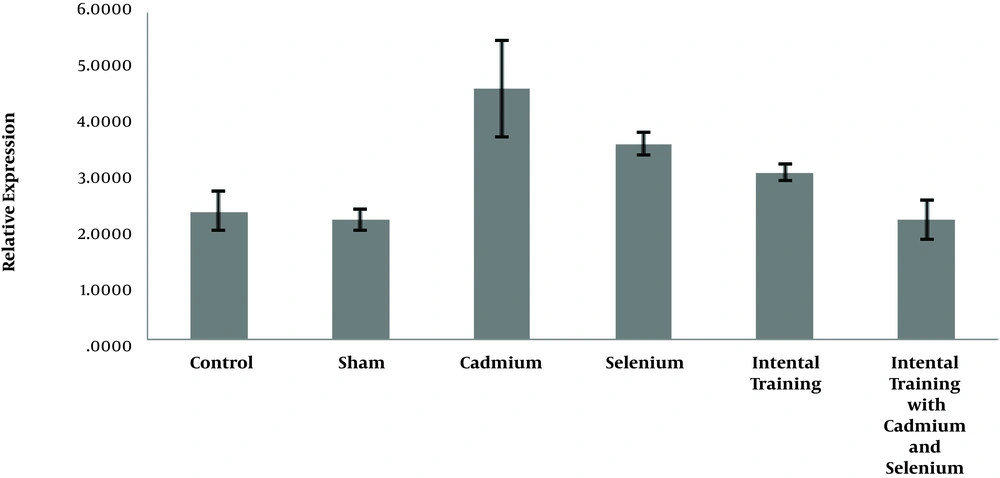
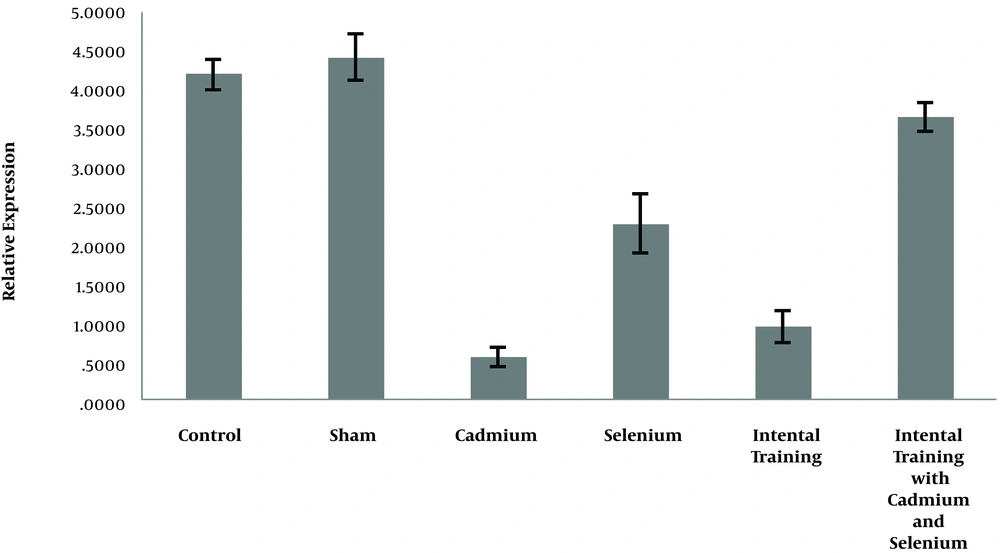
In Figures 1 and 2, the expression levels of caspase-3 and cyclin- D in liver tissue of rats are reported in six groups of the study.
4.1. Analysis the Effect of Cadmium Consumption on Caspase-3 and Cyclin-D
The results in Table 1 show that there was a significant difference between the gene expression levels of caspase-3 (P = 0.001) and cyclin-D (P = 0.001) in liver tissue of rats in the control group, sham, and cadmium. The results showed there was no significant difference in caspase-3 (P = 0.90) and cyclin-D (P = 0.29) gene expression in liver tissue of rats in the control and sham groups. However, the level of gene expression of caspase-3 in liver tissue of rats in cadmium consumption group was significantly higher than sham (P = 0.001) and control (P = 0.001) groups. Also, the level of gene expression of cyclin-D in the liver tissue of rats in the cadmium consumption group was significantly lower than the sham (P = 0.001) and control (P = 0.001) groups (Table 2).
| Sum of Squares | df | Mean Square | F | P Value | |
|---|---|---|---|---|---|
| Caspase-3 | |||||
| Between groups | 18.08 | 2 | 9.04 | 28.28a | 0.001 |
| Within groups | 3.83 | 12 | 0.32 | ||
| Total | 21.92 | 14 | |||
| Cyclin-D | |||||
| Between groups | 47.38 | 2 | 23.69 | 481.93a | 0.001 |
| Within groups | 0.59 | 12 | 0.049 | ||
| Total | 47.97 | 14 |
Results of One-Way ANOVA to Investigate the Effect of Cadmium Consumption on Caspase-3 and Cyclin-D Gene Expression in Liver Tissue of Rats
| Variable | Group | M | P |
|---|---|---|---|
| Caspase-3 | |||
| Control | Sham | 0.15 | 0.90 |
| Cadmium | -2.24a | 0.001 | |
| Cadmium | Sham | 2.40a | 0.001 |
| Cyclin-D | |||
| Control | Sham | -0.22 | 0.29 |
| Cadmium | 3.65a | 0.001 | |
| Cadmium | Sham | -3.87a | 0.001 |
Results of Tukey’s Post Hoc Test to Compare the Gene Expression Levels of Caspase-3 and Cyclin-D in Liver Tissue of Rats in the Control, Sham and Cadmium Groups
4.2. Analysis of the Effect of Interval Training and Selenium Consumption on Caspase-3 and Cyclin- D
The results showed that selenium (P = 0.001) and interval training (P = 0.001) significantly reduced caspase-3 gene expression in the cadmium-exposed rats. However, interactive effects of interval training and selenium consumption were not significant (P = 0.75). Also, selenium (P = 0.001) and interval training (P = 0.001) significantly increased the cyclin-D gene expression of cadmium-exposed rats, and the interactive effects of interval training and selenium consumption is significant (P = 0.001) (Table 3).
| Variable/Factor | Sum of Squares | df | F | P | Partial Eta Squared |
|---|---|---|---|---|---|
| Caspase-3 | |||||
| Training | 10.65 | 1 | 42.94a | 0.001 | 0.75 |
| Selenium | 4.43 | 1 | 17.86a | 0.001 | 0.52 |
| Interaction of training and selenium | 0.02 | 1 | 0.09 | 0.75 | 0.006 |
| Cyclin-D | |||||
| Training | 3.90 | 1 | 65.87a | 0.001 | 0.80 |
| Selenium | 24.59 | 1 | 415.06a | 0.001 | 0.96 |
| Interaction of training and selenium | 1.19 | 1 | 20.15a | 0.001 | 0.55 |
Results of Two-Way Variance Analysis (ANOVA) to Study the Effect of Interval Training and Selenium Consumption on Caspase-3 and Cyclin-D Gene Expression in Liver Tissue of Cadmium-Exposed Rats
5. Discussion
The results of this study showed that in the cadmium consumption group, caspase-3 was significantly higher and cyclin-D was lower than the control group, which shows that cadmium consumption has a significant effect on increase of caspase-3 and reduction of cyclin-D in liver tissue of rats. However, there were no significant differences in the gene expression levels of caspase-3 and cyclin-D of control and sham groups, which shows that in present study cadmium solvent (normal saline) had no effect on caspase-3 and cyclin-D and all changes in caspase-3 and cyclin-D were due to cadmium consumption. Cadmium can cause cell damage and apoptosis in various organs of the body, including the liver, kidneys and the heart (11), which conforms to results of this research. Also, cadmium as one of the heavy metals and environmental pollutants contributes to the induction of the Fas/Fals signal pathway and plays an important role in increasing caspase-3 (12), which is consistent with the results of this study. Also, a study reported that cadmium in the nano-molar range could increase cyclin-D and cell proliferation of the anterior pituitary tissue (13), which is not consistent with the results of this study.
Also, the results of this study showed that interval training has a significant effect on the reduction of caspase-3 and increased cyclin-D in rats exposed to cadmium. Seven weeks of swimming training resulted in decreased activity of caspase-3, as well as apoptotic agents Bax and cytochrome C, and increased Bcl2 in rats (14). In this regard, it was reported that the Bax gene expression and the Bax ratio to Bcl2, which is a factor in the production of caspase-3, was reduced in the rats that ran twelve weeks on treadmill, compared to the control group, which did not exercise (15).
Physical activity increased cell proliferation and differentiation, as well as expression of cyclin-D in neuronal cells in rats with cerebral infarction, which is consistent with the results of this study. The results of this study showed that selenium consumption has a significant effect on the reduction of caspase-3 and increase of cyclin-D in rats exposed to cadmium. The effects of selenium as an antioxidant have been investigated in various studies and it has been reported that it can reduce cellular degradation factors, including caspase-3 and Bax (16).
According to the results of this study, selenium consumption has a significant effect on the reduction of the gene expression of caspase-3 in rats exposed to cadmium. By investigating the effect of cadmium and selenium on cells, the interaction between these two in the cell can be found. The use of selenium simultaneously with the use of cadmium, due to its antioxidant properties, reduces cadmium toxicity (including reduction of caspase-3 and increased expression of PI3K/AKT/Bcl2); thus, it reduces apoptosis and preserves cellular life (17), which is consistent with the results of this research.
Concerning the interactive effects, the results of this study showed that interval training combined with selenium consumption has interactive effects on the increase of cyclin-D in rats exposed to cadmium, however, there is no interactive effect on the reduction of caspase-3. Even though in different studies, the effects of training or selenium have been investigated separately on the gene expression of caspase-3 and cyclin-D in human and other animals, including cadmium-exposed rats, no study was found to explicitly indicate the interactive effect of interval training along with selenium consumption on the level of caspase 3 and cyclin-D in rats exposed to cadmium, so that the results could be compared with the present study; therefore, in order to fully understand the interactive effects of exercise training and selenium consumption on caspase-3 and cyclin-D in cadmium-exposed rats, more studies are needed in this regard.
5.1. Conclusions
According to the findings of the present research, it seems that interval training and selenium consumption alone leads to improvement of caspase-3 and cyclin-D in liver tissue of rats exposed to cadmium. Also, interval training with selenium consumption has interactive effects on the improvement of hepatocyte apoptosis in rats exposed to cadmium.