1. Background
Hepatitis B virus (HBV), which contains double-stranded DNA, is a species of the genus Orthohepadnavirus and a member of the family Hepadnaviridae (1). Its genome consists of four open-reading frames (ORFs), including the largest ORF (Pol) encoding viral polymerase, surface proteins (S-ORF) encoding three carboxy-terminal HBV surface (HBs) proteins, and precore/core (preC/C) ORF encoding the structural core protein, as well as an extra precore sequence. Precore/core protein expression results in the synthesis and subsequent proteolytic processing of a non-structural form of core protein, known as hepatitis B e-antigen (HbeAg). The X-ORF is encoded by X protein, as a non-structural protein, and is highly associated with the epigenetic control of HBV transcription (2). There are ten known genotypes (A-J) for HBV. The majority of these genotypes can be divided into subgenotypes, with certain virologic and epidemiological properties (3, 4).
Hepatitis B virus can lead to acute or chronic hepatitis and liver cancer and is recognized as one of the global health problems (5). Reuse of syringes and needles, unsafe blood transfusion, shaving from barbers, tattooing, and surgical instruments are the major causes of HBV transmission (1). The prevalence of HBV, which varies geographically, is unknown in some regions, as it can remain asymptomatic for a long period before the emergence of chronic infection-related complications. According to recent statistics, 350 million individuals have been chronically infected with HBV worldwide (3, 6). The average prevalence for anti-HBc antibodies is 16.4% in Iran (7). Hepatitis delta virus (HDV) is known as a small, single-stranded, defective RNA virus, which requires HBV for complete replication and transmission. It is covered by the envelope protein of pre-S and S antigens in HBV (8). Hepatitis D virus RNA contains six ORFs. One ORF is encoded for hepatitis delta antigen (HDAg), whereas other ORFs may not be transcribed (9). There are two HDAgs, including small HDAg, which enhances genome synthesis, and large HDAg, which prevents HDV RNA synthesis and is essential for the virion morphogenesis (8, 10).
Hepatitis D virus infection can lead to several liver diseases in humans worldwide (11). Infection with HDV and HBV occurs simultaneously with and/or after initial HBV infection (12). Hepatitis B virus /HDV coinfection can result in more severe diseases and cause a higher risk of mortality (13). Hepatitis D virus is transmitted in a similar mode to HBV (10). Hepatitis D virus has been reported in more than 18 million people with evidence of exposure to HDV, who have been already infected with HBV (14). Hepatitis D virus infection is endemic to the Middle East (e.g., Iran), Central Africa, Horn of Africa, Mediterranean countries, Eastern Europe, Amazon basin and some Asia countries (12). In a systematic review, the overall HDV seropositivity rate of 6.61% among Iranian patients with chronic HBV infection (15). Recently, two types of drugs have been introduced for the treatment of chronic HBV infection: the immune modulator interferon-α (standard/ pegylated (PEG)-INF-α) and nucleoside/ nucleotide analogs, such as lamivudine (LAM), adefovir dipivoxil (ADV), telbivudine (LdT), entecavir (ETV) and tenofovir disoproxil fumarate (TDF). Compared to natural substrate deoxyadenosine triphosphate (dATP), nucleoside/nucleotide analogs can prevent HBV replication and terminate HBV DNA chain extension (9).
Lamivudine is the most commonly used analog type for patients with chronic HBV infection. On the other hand, there is a possibility of drug resistance, and mutations may occur in the polymerase gene of the virus. As previous studies have shown, this drug is associated with mutations in the YMDD motifs, substitution of methionine by valine (rtM204V-YVDD) or isoleucine (rtM204I-YIDD), and or rarely serine (rtM204S-YSDD) in the tyrosine-methionine aspartateaspartate (YMDD) motif of the HBV polymerase gene (16, 17). Serological tests, including rapid diagnostic tests (RDTs), enzyme-linked immunosorbent assay (ELISA), and DNA detection methods, such as nested PCR and real-time PCR assays, are commonly used to diagnose HBV (6). The nested PCR is a modified PCR method, developed for improving sensitivity and specificity. In this method, two primer sets and two successive PCR reactions are used to detect low viral loads (18, 19).
2. Objectives
This study aimed at determining the prevalence of HBV/HDV co-infection and investigating the YMDD motif in HBV-infected patients.
3. Methods
3.1. Patients and Specimens
In this cross-sectional study, a total of 1600 blood specimens were collected from HBV-suspected patients, who were referred to the diagnostic centers of Qom Province, Iran from July 2018 to March 2019 before and after lamivudine therapy. The sample size required for the study with 95% confidence interval and an expected prevalence of 50% was calculated based on Cochran’s formula.
3.2. Detection of HBV by ELISA Method
Blood specimens were tested for hepatitis B surface antigen (HbsAg) detection by ELISA method using an ELISA kit (Pishtazteb, Iran) (1).
3.3. Viral DNA and RNA Extraction and PCR Assay
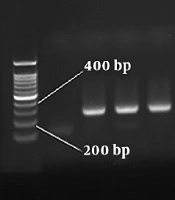
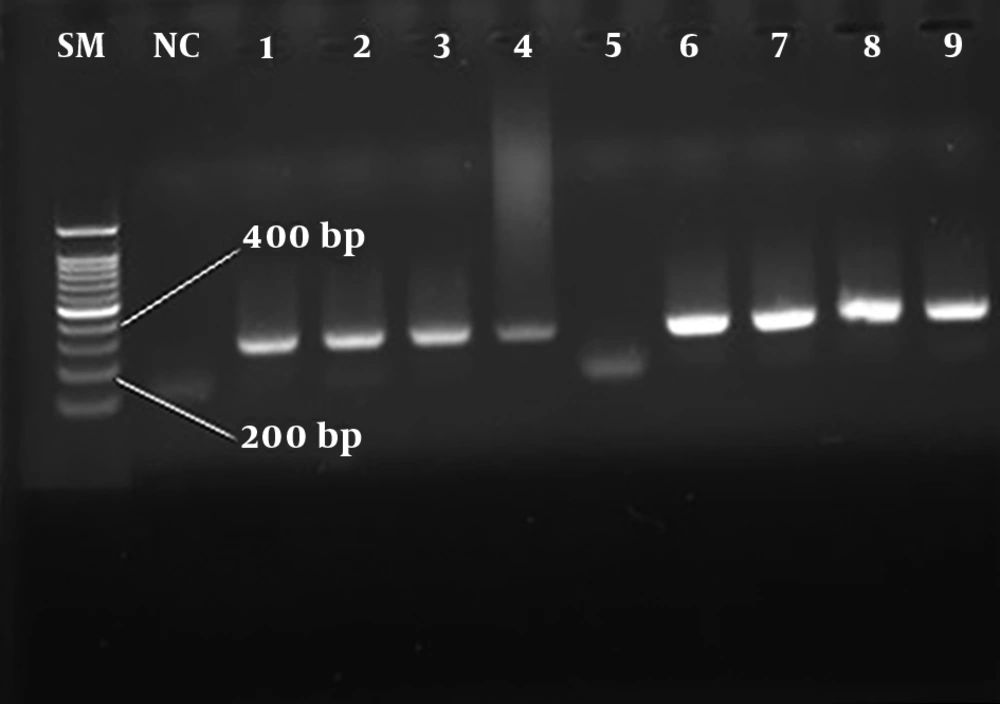
In order to confirm the presence of HBV DNA in the blood specimens of HbsAg-positive patients, nested PCR technique was applied. A genomic DNA extraction kit (Sinaclon, Iran) was used to extract HBV DNA based on manufacturer’s protocol. The primers were designed for the HBs gene of HBV using GenRunner and CLC Sequence Viewer 6 (Table 1). The positive control was blood serum infected with HBV, and serum-derived DNA from HBV-negative patient was employed for negative control. Table 2 indicates the instructions used in the first and second rounds of PCR for HBs gene. Moreover, a genomic RNA extraction kit (Vivantis, Malaysia) was used for the extraction of HDV RNA. Complementary DNA (cDNA) synthesis (Vivantis, Malaysia) was performed following the manufacturer’s instructions.
| Gene | Primer Sequence | Product Size |
|---|---|---|
| HBs | Outer primer | 303 bp |
| F: 5’-GGCGCACCTCTCTTTACG-3’ | ||
| R: 3’-ATAGGGGCATTTGGTGGTC-5’ | ||
| Inner primer | ||
| F: 5’-CACTTCGCTTCACCTCTGC-3’ | ||
| R:3’-CCAAGGCACAGCTTGGAGGCTTGAA-5’ | ||
| HDAg | Outer primer | 405 bp |
| F: 5’-CCAGGTCGGACCGCGAGGAGG | ||
| R: 3’- ACAAGGAGAGGCAGGATCACCGAC | ||
| Inner primer | ||
| F: 5’- GATGCCATGCCGACCCGAAGA | ||
| R: 3’- GAAGGAAGGCCCTCAAGAACAAGA | ||
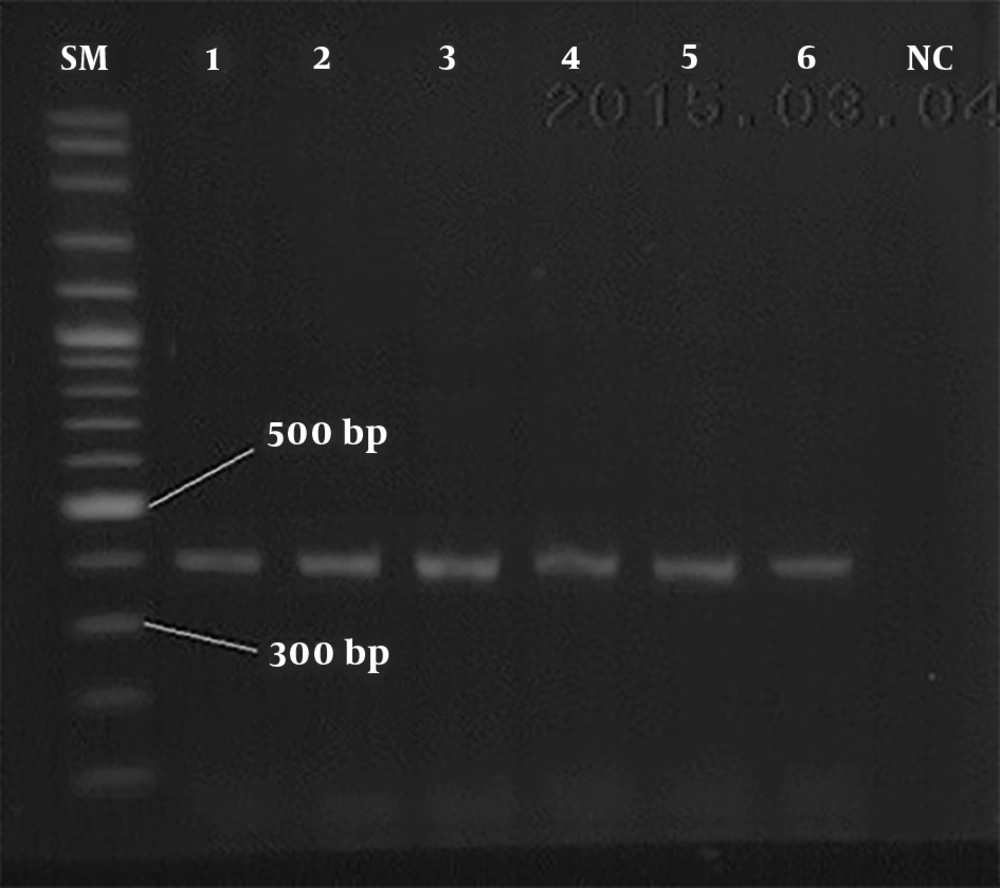
| YMDD | F: 5’-TCTTCTTGTTGGTTCTTCTGGAC-3’ | 591 bp |
| R: 3’- GCAAAGCCCAAAAGACCC -5’ |
| Gene | Initial Denaturation | Subsequent Denaturation | Annealing | Extension | Final Extension |
|---|---|---|---|---|---|
| HBs | |||||
| First round | 95°C 3 min 1x | 95°C 30 s 35x | 50°C 30 s 35x | 72°C 45 s 35x | 72°C 5 min 1x |
| Second round | 95°C 3 min 1x | 95°C 30 s 35x | 53°C 30 s 35x | 72°C 45 s 35x | 72°C 5 min 1x |
| HDAg | |||||
| First round | 95°C 3 min 1x | 95°C 1 min 34x | 55°C 1 min 34x | 72°C 1 min 34x | 72°C 10 min 1x |
| Second round | 95°C 3 min 1x | 95°C 1 min 34x | 62°C 1 min 34x | 72°C 1 min 34x | 72°C 10 min 1x |
Abbreviations: °C, Celsius; min, minute; x, cycle of reaction; s, second.
All specimens were tested by PCR to detect YMDD motif. YMDD motif amplification was performed by initial denaturation (95°C - 4 minutes); subsequent denaturation (95°C - 45 seconds); 35 cycles of annealing (52°C - 30 seconds); extension (72°C - 30 seconds), and final extension (72°C - 5 minutes). Each reaction mixture (5 µL) was run on 1% agarose gel with Safe Stain (GelRed®, California) in a UV transilluminator. In order to confirm the presence of HDV HDAg gene, the PCR products of HBV/HDV-positive specimens were subjected to direct sequencing.
3.4. Statistical Analysis
SPSS version 22.0 and Microsoft Excel version 2016 were used for data analysis. The level of significance in t-test was set at P < 0.05.
4. Results
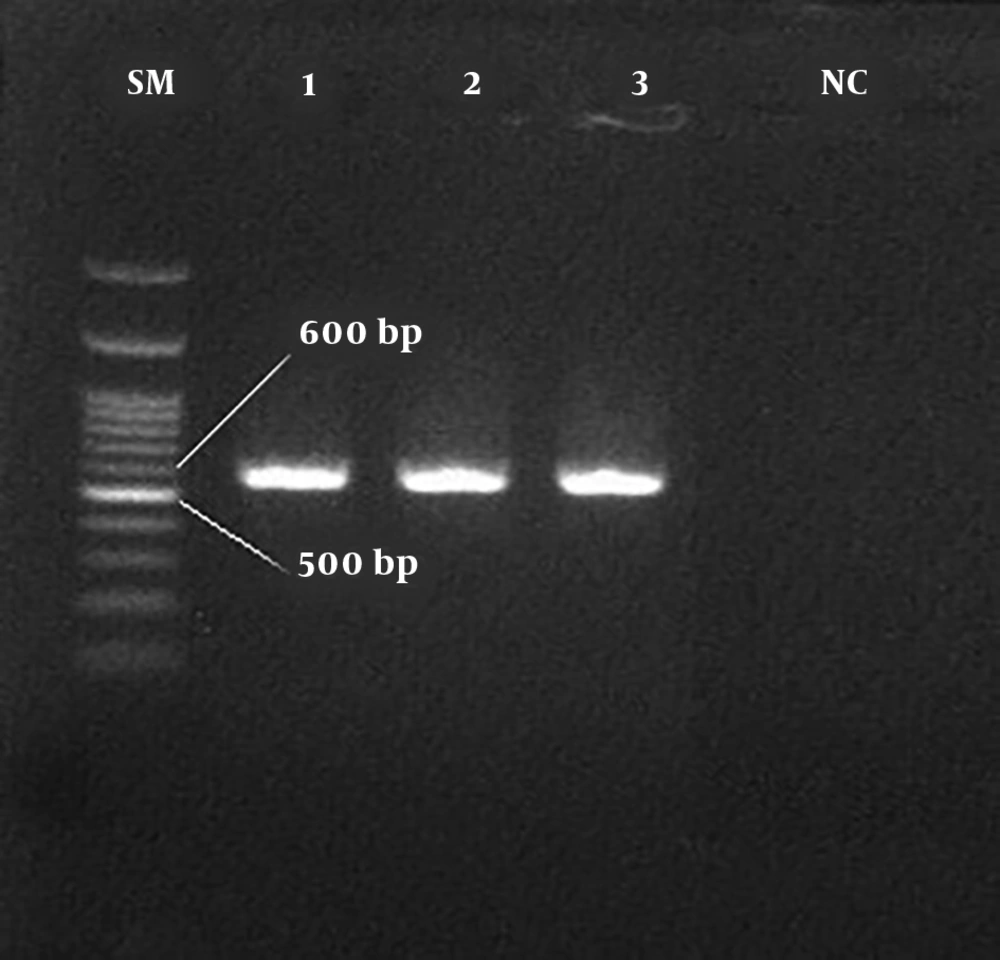
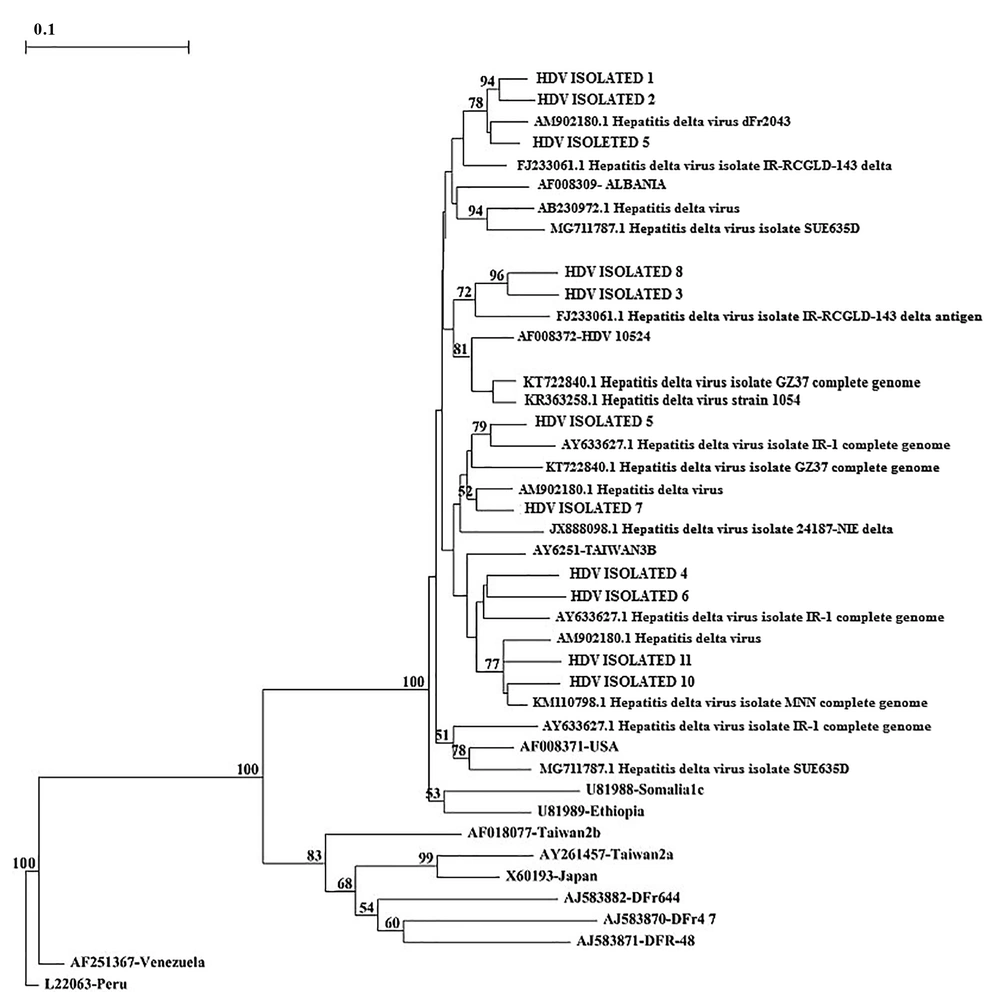
In this study, a total of 1600 blood specimens were collected from HBV-suspected patients (age range: 20 - 67 years), who were referred to the diagnostic centers before and after lamivudine therapy of Qom province. The number of HBsAg positive patients was 180 (11.25%) (P < 0.05) based on ELISA and nested PCR techniques. In total, 121 out of 980 men and 59 out of 692 women were HBsAg positive with the mean age of 32 - 38 years. Moreover, 9 men (5%) and 2 women (1.1%) were infected with HBV/HDV. Table 3 presents a statistical comparison of HBV-suspected patients. The amplification results of HBV HBs gene and HDV HDAg gene by nested PCR technique are shown in Figures 1 and 2, respectively. The frequency of lamivudine resistance mutation in the YMDD region was 28 (15.5%) in chronic HBV patients. Figure 3 indicates the amplification of YMDD motif based on PCR technique. The constructed phylogenetic trees for HDAg gene of HDV showed that most HBV/HDV isolates had similar sequences to the Iranian isolates (Figure 4).
| HBsAg Positive, No. (%) | HBV/HDV Positive, No. (%) | |
|---|---|---|
| Total | 180 (11.25) | 11 (6.1) |
| Gender | ||
| Male | 121 (7.5) | 9 (5) |
| Female | 59 (3.6) | 2 (1.1) |
| Marital status | ||
| Single | 83 (46.1) | 3 (27.2) |
| Married | 97 (53.9) | 8 (72.7) |
| Abdominal pain | 28 (15.5) | 2 (18.2) |
| Fever | 18 (10) | 3 (27.2) |
| Loss of appetite | 20 (11.1) | 4 (36.3) |
| Drug users | 10 (5.5) | 1 (9) |
| Surgery | 12 (6.6) | 2 (18.2) |
| Tattooing | 31 (17.2) | 5 (45) |
| Blood transfusion | 26 (14.4) | 4 (36.3) |
5. Discussion
Hepatitis B virus and HDV infections are known as major health issues, leading to cirrhosis and hepatocellular carcinoma (20). The outcomes of HBV infection are clinically diverse ranging from the asymptomatic carrier state to progressive chronic HBV infection (21). Hepatitis D virus only spreads in human hepatocytes and/or in the presence of HBV, leading to fulminant or chronic hepatitis with liver cirrhosis (22). The average prevalence of HBV antigen was estimated at 2.4% in Pakistan (23), 88.8% in Brazil (3), 37.5% in Tehran province (Iran) (17), 5.88% in Bandar Abbas province (Iran) (24), 11% Hormozgan province (Iran) (7), and 6.72% in Bushehr province (Iran) (25). However, in the present study conducted in Qom province (Iran), 180 (11.25%) out of 1600 patients were HBsAg positive and 11 patients (6.1%) were found to be positive for dual infection (HBV/HDV).
Studies have shown that chronic HDV infection associated with more severe liver diseases in comparison with chronic HBV monoinfection (9). The prevalence of HDV infection has been reported 2% - 20% among HBsAg carriers, and it is endemic to some cities of Iran (11, 26). The prevalence of HDV was 2.1% in Southern China (27), 6.5% in Guangzhou (14), 15.3% in Taiwan (28), 10.85% in Pakistan (29), 7.7% in Tehran province (12), 5% Hormozgan province (Iran) (7), 2% in Qom province (30), 2.9% in Isfahan province (Iran) (31), and 3.1% -3.3% in Birjand province (Iran) (32, 33). According to statistics, the prevalence of HDV has increased in Iran.
On the other hand, over the past two decades, considerable progress has been made in the treatment of chronic HBV infection due to the development and availability of nucleotide analogs, such as lamivudine. However, patients have developed drug-resistance due to the widespread use of lamivudine worldwide (34). The mechanism of action of lamivudine is mutation within the HBV polymerase gene (9). According to several Iranian studies, the frequency of lamivudine resistance mutations in the YMDD region is 86.6% (35), 40% (17), 26.7% (36) and 5.3% (16) in Iran. The YMDD frequency was reported to be 58.72% in China (37), 35% in Egypt (38) and 67% in Brazil (3), while in the present study, its frequency was estimated at 15.5%, which is in agreement with the YMDD frequency reported by Khojareh et. al (17.74%) (39).
Caution should be taken with regard to the comparison of this study with previous research due to the use of different methods of detection and sample size. The current study was performed for the first time in Qom province, Iran. This province is a major destination for travelers and immigrants, most of whom come from different provinces of Iran or African and Asian countries, which are endemic to many diseases and have poor healthcare systems. The nested PCR technique includes two primers sets which are applied in two successive runs of PCR. The second set amplifies a secondary target through the first run product and limits non-specific products (40). Our results showed that nested PCR is a highly sensitive technique.
5.1. Conclusions
Early diagnosis of chronic HBV and HDV infections using the nested PCR technique is essential in high-risk groups for infection control. Considering the rapid increase in knowledge about the frequency of chronic HBV and HDV infections and development of new antiviral medicines, treatment guidelines are changing rapidly. Also, appropriate treatment decreases the mortality rate due to liver disease.