1. Background
In Iran, Leishmania infantum parasites cause visceral leishmaniasis that is transmitted by the bites of infected Larrossious sandflies (1). Ardabil, East Azerbaijan, Fars, Bushehr, Kerman, Qom, and North Khorasan provinces are known as the endemic foci of visceral leishmaniasis in Iran (2). Domestic canines (Canis familiaris) that have close contact with humans are the main reservoir hosts of visceral leishmaniasis (3-5). Based on previous studies, the owners of infected dogs and their families (generally under the age of 10) are more exposed to infection (6, 7). Visceral leishmaniasis is acknowledged as one of the 17 “neglected tropical diseases” by the World Health Organization (8-10) due to being a chronic and often fatal zoonosis causing a public health problem. Despite many attempts, no preventive drug or vaccine exists for it (11).
Canine visceral leishmaniasis appears in a wide range from asymptomatic to acute and fatal forms (12, 13), with unspecific, variable, multisystemic sings (14). Cachexia, ocular lesion, anemia, diarrhea, weight loss, anorexia, epistaxis, dermatitis, alopecia, lymphadenopathy, and cutaneous ulcerations are the clinical manifestations of infected dogs (15-17). Asymptomatic sheepdogs as permanent and unnoticed reservoirs in endemic areas can affect the populations of infected dogs and humans through sandflies. These seropositive asymptomatic dogs can develop clinical signs throughout their lives (14, 18). Furthermore, male dogs can infect female dogs through the semen during mating (8, 19).
The crucial way to control visceral leishmaniasis in endemic and non-endemic regions is the early detection of asymptomatic carriers (14, 20). Molecular methods, unlike serological tools, can firmly discriminate Leishmania species. Sensitivity and specificity of molecular tools have been assessed in visceral leishmaniasis diagnosis in both canines and humans with variable values (21-23). The specificity of molecular tests is often 95% to 100% and its sensitivity can be between 0.001 and 0.1 parasite/reaction (7, 24, 25). The ITS-rDNA gene is considered the most suitable gene to determine phylogenetic affinities among Leishmania spp. and resolve the taxonomic status of parasites in the old world. It is located between the 18S and 5.8S rRNA genes. In addition, it includes conservative loci targets and acceptable polymorphisms to enable species identification (5, 26, 27). The source of obtained samples is important in the accuracy of detecting infected canines. Blood samples, swab rubbing of oral mucosa/snout, and the conjunctiva provide high loads of parasites and harvesting these samples is less invasive than lymph node and bone marrow sampling (28, 29).
2. Objectives
The objective of this study was to detect the causative agents of visceral leishmaniasis in asymptomatic dogs in North Khorasan using less invasive and more sensitive diagnostic methods.
3. Methods
3.1. Study Area
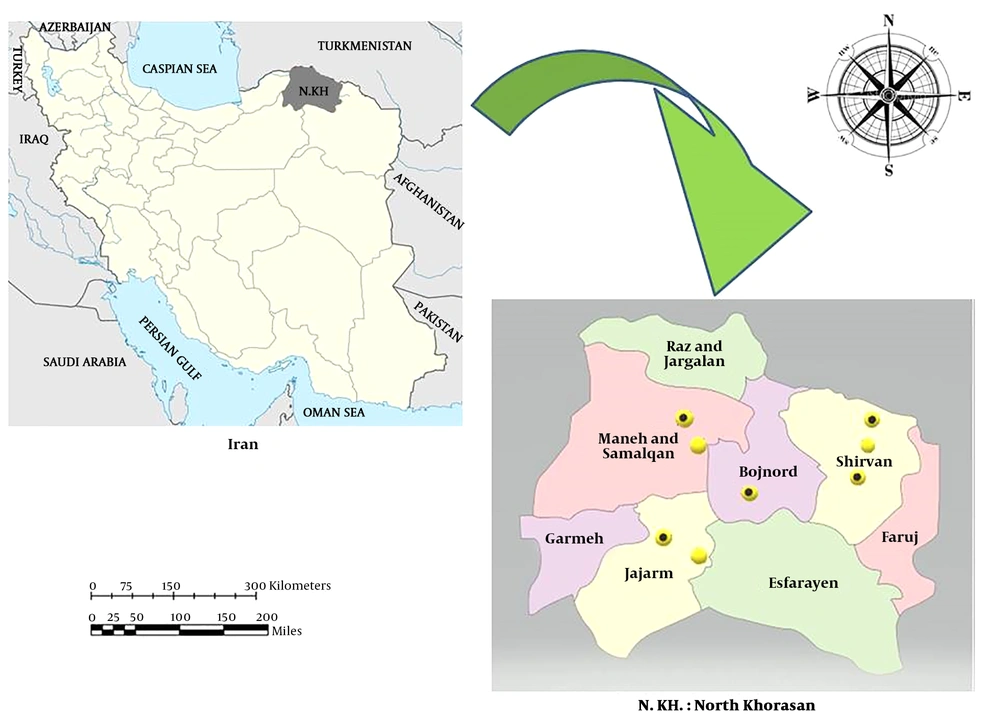
North Khorasan Province is located in Northeastern Iran with coordinates of 37.4761°N and 57.3317°E and an average altitude of 1,326 meters above the sea level (m asl). The province has seven districts including Bojnourd, Shirvan, Jajarm, Raz, Jargalan, Maneh, and Samalghan. Sampling was done from 28 July to 4 August 2018 in different villages of the mentioned areas. The sampling villages were chosen based on data collected from healthcare and veterinary authorities of the province along with the reports of Sheikh Hospital of Mashhad City to which most visceral leishmaniasis patients are sent for diagnosis and cure (Figure 1).
3.2. Sample Collection
The asymptomatic sampled canines were chosen randomly from sheepdogs. For the first time, a non-invasive sampling method without anesthesia was performed in this province. Three different samples including a blood sample using a vacuum tube, a swab sample from the snout, and a swab sample from the right and left conjunctiva, in sequence, were taken from each dog using only non-invasive methods. Sterile cotton swabs were rubbed against the lower eyelid surface and inside the lower lip of each dog; then, the cotton tip was put in a sterile tube containing PBS medium (30). Blood samples were also taken from each dog from the cephalic vein using a vacuum tube containing EDTA. All specimens were, then, stored first at 4°C in the field and later at -20°C in the Molecular Systematics Laboratory of Pasteur Institute of Iran. Detailed information including sex, age, breed of each sample, their owners, and the location was gathered in pre-designed forms. Each protocol and method applied in this survey followed the principles of the Declaration of Helsinki (31).
3.3. DNA Extraction and Single-Round Polymerase Chain Reaction (PCR)
The DNA of parasites was directly extracted from the whole blood, PBS containing swabs based on the modified phenol-chloroform protocol (32-34). The single-round PCR was employed to detect Leishmania parasites in suspected dogs by targeting ITS-rDNA at about 480 bp. The amplification reaction was carried out in a total of 20 µL volume containing 1× Taq polymerase buffer, 1.5 mM MgCl2, 60 µM of each dNTP, one unit Taq polymerase (pre-prepared 2× Amplicon master mix), 1 µM primer ITS1F (5’GCAGCTGGATCATTTTCC3’), 1 µM primer ITS2R4 (5’ATATGCAGAA GAGAGGAGGC3’), and 1.5 µL (5 - 10 ng/µL) of the DNA extracted from samples. The mixture was incubated in a master cycler gradient Eppendorf Thermocycler (0.2 mL block) at 94°C for 5 min, followed by 37 cycles each consisting of 45 s at 94°C, 45 s at 60°C and 30 s at 72°C. After the last cycle, an extension was applied for a further 10 min and then it was held at 4°C. The PCR products were subjected to electrophoresis in the 1.5% agarose gel and observed under ultraviolet light after staining for 15 min with 0.5 g/mL safe staining. The PCR amplification was performed for the Leishmania-specific ribosomal internal transcribed spacer 1 (ITS1) region using the primer pairs of ITS1F and ITS2R4. For positive control, the extracted DNA of L. infantum (Lon 49) and L. tropica (MHOM/IR/02/Mash10) was used (35, 36).
3.4. Sequencing and Phylogenetic Analysis by Targeting ITS-rDNA
The PCR products were directly sequenced by targeting the ITS-rDNA gene using the ITS1F and ITS2R4 primers (Pishgam Biotech Co., Tehran, Iran). Individual sequences were aligned and edited in consensus positions compared to the GenBank sequences of all regional species using Sequencher V. 4.1.4 software for PC. The maximum likelihood (ML) tree was constructed via MEGA V. 5.05 for showing the phylogenetic position of common and new haplotypes of the ITS-rDNA sequences based on the Kimura 2-parameter model.
4. Results
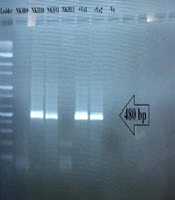
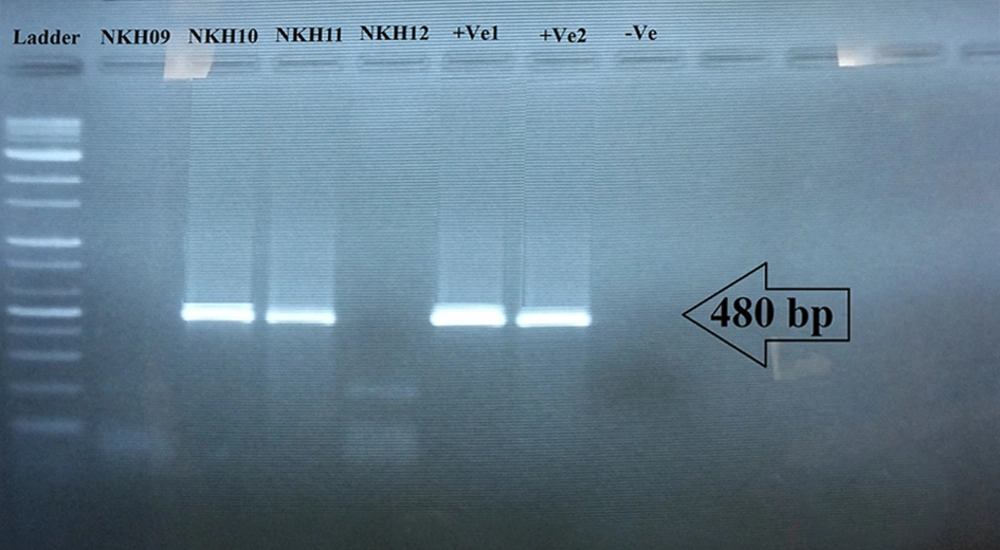
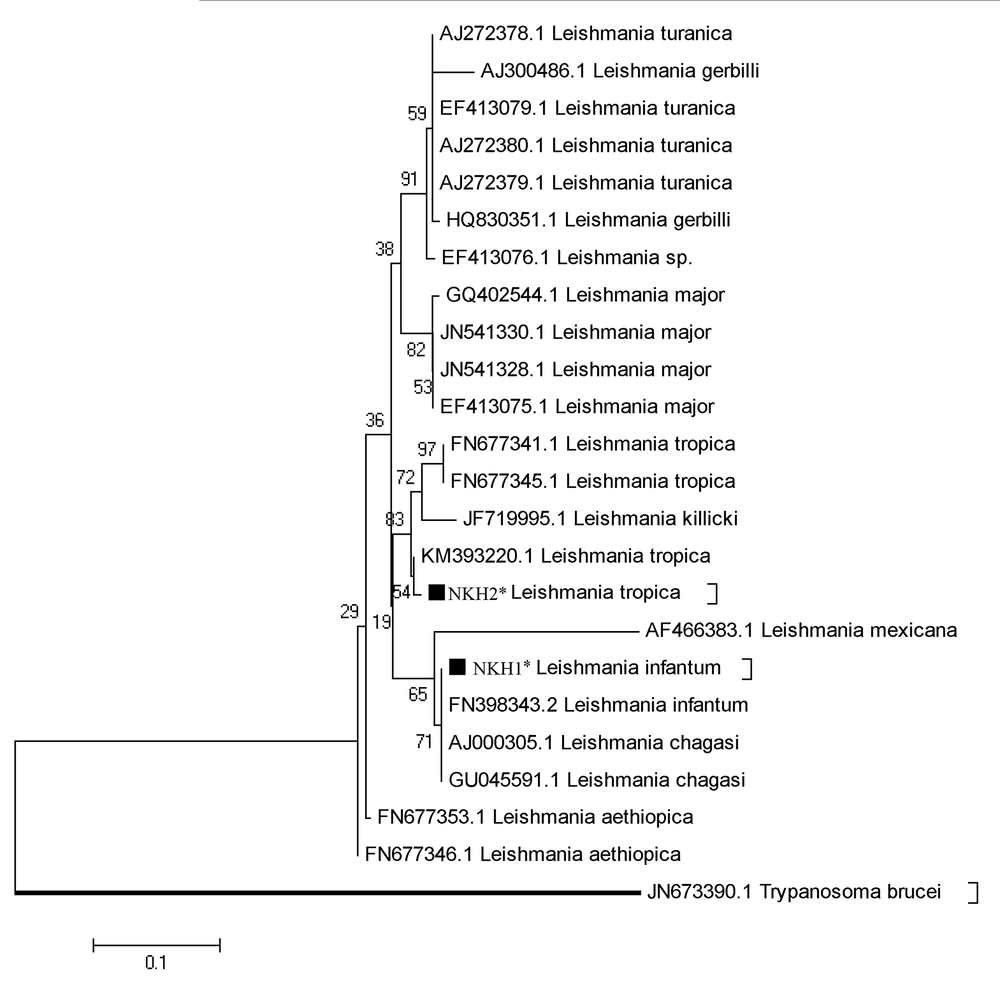
Of 37 sampled dogs, 22 (59.45%) were less than five-years-old, 13 (35.13%) were five to 10-years-old, and two (5.40%) were older than 10 years (Table 1). A 480 bp fragment was successfully amplified to detect Leishmania spp. It was shown that 11/37 (29.73%) asymptomatic dogs were infected with Leishmania. Since all Leishmania-positive samples showed a 480 bp amplified DNA band on the agarose gel (Figure 2), all the 11 samples of infected dogs were sent for sequencing. After editing and alignment, a phylogenic tree was constructed using GenBank-submitted sequences including “KM393220.1 for L. tropica”, “FN398343.2 as L. infantum”, and “EF413075.1 as L. major” while for the out-group, Trypanosoma brucei with the accession number of “JN673390.1” was employed. Based on the phylogenetic analysis, 10 dogs were definitely diagnosed with L. infantum (NKH1*) and one dog was infected with L. tropica (NKH2*) (Figure 3). Three of them were less than five-years-old (27.27%), six were between five and 10 (54.54%), and two were older than 10 (18.18%) (Table 1). Among the 11 infected canines, 21 (17.8%) out of 118 collected samples from blood (n = 8; 38.09%), snout (n = 3; 14.28%), right conjunctiva (n = 6; 28.57%), and left conjunctiva (n = 4; 19.04%) were infected with the Leishmania parasite. None of the 11 dogs were simultaneously infected in all the assessed samples (blood, snout, right and left conjunctiva). However, in three dogs, the infection was found in three out of four sampled areas, two of which had infections in their right and left conjunctiva and blood and one of them had an infection in its conjunctiva, snout, and blood concurrently (Table 1).
| Location | Village | Sampled Dogs, % | Code Number | Age | Sex | Infected Dogs | Molecular methods (PCR and Sequencing) | Total Collected Samples, % | Total +Ve (LC, RC, S, and B), % | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LC | RC | S | B | |||||||||
| Shirvan | Chalu | 4 (10.8) | NKH01 | 5 y | M | * | +Ve | +Ve | 16 (13.5) | 2 | ||
| NKH02 | 12 y | M | * | +Ve | +Ve | 2 | ||||||
| NKH03 | 6 m | M | * | +Ve | +Ve | 2 | ||||||
| NKH04 | 2 y | M | * | +Ve | +Ve | +Ve | 3 | |||||
| Old Oghaz | 10 (27) | NKH09 | 2.5 y | M | 40 (33.8) | |||||||
| NKH10 | 6 m | M | * | +Ve | 1 | |||||||
| NKH11 | 6 y | M | * | +Ve | +Ve | +Ve | 3 | |||||
| NKH12 | 1.5 y | M | ||||||||||
| NKH13 | 1 y | M | ||||||||||
| NKH14 | 7 m | M | ||||||||||
| NKH15 | 4 y | M | ||||||||||
| NKH16 | 1.5 y | F | ||||||||||
| NKH17 | 1.5 y | F | ||||||||||
| NKH18 | 2 y | F | ||||||||||
| Zortanlu | 4 (10.8) | NKH19 | 5 y | M | 16 (13.5) | |||||||
| NKH20 | 5 y | M | ||||||||||
| NKH21 | 5 y | M | ||||||||||
| NKH22 | 4 y | M | ||||||||||
| Bojnurd | Peighu | 4 (10.8) | NKH05 | 5 y | M | * | +Ve | +Ve | +Ve | 16 (13.5) | 3 | |
| NKH06 | 8 mo | M | ||||||||||
| NKH07 | 5 y | F | * | +Ve | +Ve | 2 | ||||||
| NKH08 | 12 y | M | * | +Ve | 1 | |||||||
| Jajarm | GharajeRobat | 5 (13.5) | NKH23 | 1.5 y | M | 17 (14.4) | ||||||
| NKH24 | 2 y | M | ||||||||||
| NKH25 | 8 y | M | ||||||||||
| NKH26 | 1.5 y | M | ||||||||||
| NKH27 | 10 y | M | * | +Ve | 1 | |||||||
| Emarat | 3 (8.1) | NKH28 | 5 y | F | 3 (2.5) | |||||||
| NKH29 | 3 y | M | ||||||||||
| NKH30 | 5 y | F | ||||||||||
| Maneh and Samalghan | Ashkhaneh | 3 (8.1) | NKH31 | 3 y | M | 3 (2.5) | ||||||
| NKH32 | 4.5 y | M | ||||||||||
| NKH33 | 3 y | M | ||||||||||
| Darkesh | 4 (10.8) | NKH34 | 4 y | M | 7 (5.9) | |||||||
| NKH35 | 10 y | M | * | +Ve | 1 | |||||||
| NKH36 | 6 y | M | ||||||||||
| NKH37 | 4 y | M | ||||||||||
| Total | 37 | 37 | 11/37 (29.7%) | 4 | 6 | 3 | 8 | 118 | 21/118 (17.8%) | |||
Abbreviations: B, blood; F, female; LC, left conjunctival; M, male; mo, month; RC, right conjunctival; S, snout; +Ve, positive sample; y, year.
aValues are expressed as No. (%).
The phylogenetic tree of Leishmania species according to the maximum likelihood (ML) method conducted based on the multiple sequence alignment of the ITS-rDNA gene by MEGA5.05. Trypanosoma brucei (JN673390) was considered as the out-group branch.∗, Identified sequences in this study for L. infantum and L. tropica complexes.
5. Discussion
Until now, studies on visceral leishmaniasis in dogs in North Khorasan Province were regional and did not survey the entire province, unlike the current study. Also, most research has been on canines with clinical symptoms via serological methods. Even if molecular methods were applied, they were on symptomatic and seropositive dogs. Furthermore, the studies were all done on blood and bone marrow samples and other organs were not tested (37-39).
Villagers of North Khorasan Province are generally herdsmen and their sheep and cattle flocks along with their sheepdogs are kept in the vicinity of residential areas and shelters. On the other hand, houses and groves are made of clay, which makes a good environment for the propagation of sandflies. Also, sheepdogs are always kept outside during the day and night. Most dogs were asymptomatic and under favorable conditions of visceral leishmaniasis, they are potential reservoirs of the disease, causing the spread of the disease. Besides, even if dogs had signs, their owners would not take effective actions due to the lack of knowledge of canine visceral leishmaniasis, its preventive factors, transmission, and treatment. Usually, female dogs are sent to ranches with the herd and even if they are not infected by sandfly bites, they may be contaminated by mating with an infected male, which causes an increase in the population of asymptomatic infected dogs (8).
Children under the age of five are more susceptible to visceral leishmaniasis. This could be due to the fact that their immune systems are more immature than those of adults. Based on the medium of canine longevity (40), the risk of infection, development, and conversion of asymptomatic visceral leishmaniasis to symptomatic leishmaniasis will decline due to an increase in cell-mediated immunity associated with age (41, 42). Based on data obtained from Sheikh Hospital of Mashhad, from 15 reported visceral leishmaniasis cases during 2006 - 2017, 10 cases were males and five were females. Of these, 13 were less than five-years-old and two were over five-years-old. Of these samples, eight were reported during spring and summer and seven were recorded in autumn and winter. Moreover, five cases (33.33%) were from Bojnord District, two cases (13.33%) from Raz and Jargalan District, four cases (26.66%) from Maneh and Samalghan, and four cases (26.66%) from Shirvan District.
Despite the visceral leishmaniasis infection age distribution throughout Iran in previous studies (12), our findings showed that most of the studied dogs were less than five-years-old, but the highest number of infections was in the age group of 5 - 10 years, which probably indicates that this age group is more sensitive to visceral leishmaniasis in this region. Although the bone marrow and lymph nodes have many parasites, according to studies, conjunctiva, oral mucosa or snout and blood are both reliable and suitable for molecular studies because the sample source in dogs is important for accurately determining the contamination. In addition, this method is non-invasive and ethical and the animal is less harassed and ultimately survives (28, 29). Leishmania-positive dogs can be treated and followed up; also, visceral leishmaniasis preventive measures can be taken to reduce the infection risk.
By employing the sensitive and specific ITS-rDNA gene (36), approximately 30% of the sampled dogs were found to be infected with L. infantum (n = 10), as the most important recognized causative agent of visceral leishmaniasis in dogs (23, 43, 44), as well as L. tropica (n = 1). Nearly 50% of the infected specimens were found in conjunctival samples, which were significantly more infected than blood samples. Our findings are confirmed by previous studies. Strauss-Ayali et al. (30) in 2004 detected Leishmania infection from conjunctiva in 83% of the sampled canines using ITS1. Also, Ferreira et al. (45) in 2008 used the kDNA gene to detect 74% infection in dogs’ conjunctiva. In this survey, based on the results of sequencing, L. infantum was confirmed as the most causative agent of visceral leishmaniasis in North Khorasan Province. Also, one dog was found to be infected with L. tropica. All the infected canines were asymptomatic. Leishmania tropica (NKH2) found in our study was close to a haplotype found in Khouzestan Province, Iran (Spotin et al. 2014) and L. infantum (NKH1) was close to the haplotype submitted by Santos-Oliveira et al. in 2011 (46, 47).
Two separate studies done by Hajjaran et al. (4, 48) in 2007 and 2013 also detected L. tropica from Iranian canines in endemic regions of visceral leishmaniasis. Baneth et al. (49) in 2017 reported L. tropica and even L. major from infected dogs in Palestine. Research indicates that L. tropica infection usually causes skin lesions, pustulat dermatitis splenomegaly, and lymphadenomegaly, which are similar to canine viscera-cutaneous leishmaniasis caused by L. infantum. Like in human cases, though L. major infections only can cause cutaneous diseases, L. tropica can have a role in human visceral leishmaniasis. In some cases, parasite culture and serological surveys can be negative for leishmanial infections; therefore, various forms of PCR can be performed to accurately detect any Leishmania spp. infections in blood samples (49).
Several strategies have been proposed for preventing and controlling canine visceral leishmaniasis. Infection prevention can be achieved by insecticide products (e.g., deltamethrin, permethrin, flumethrin, and fipronil), but in Iran, the use of insecticide-impregnated collars can be more effective to significantly reduce the disease among dogs because killing healthy and seropositive (asymptomatic) dogs is immoral for controlling the disease, disrupts control programs, and reduces their effect (7, 50-52). Studies showed that deltamethrin released from Scalibor collars reduced the blood-feeding of sandflies by 90% and increased the mortality rate of those who had fed by 35% - 100%. The findings show that Scalibor collars can give 94% - 98% annual protection when facing sandfly bites (41, 53-55).
5.1. Conclusions
This study gave us an overview of the epidemiology of this disease. It also confirmed the local presence of canine visceral leishmaniasis and showed the distribution of contamination in asymptomatic dogs throughout this province as an endemic area of visceral leishmaniasis. Our study showed that based on the 480 bp ITS-rDNA gene and phylogenic analysis, different species of Leishmania parasite were firmly detected, differentiated, and accurately identified. The current findings indicate that the non-invasive sampling methods are more reliable and suitable than lymph node and bone marrow sampling in the field condition to identify visceral leishmaniasis. This is the first report of the visceral involvement of a shepherd dog with L. tropica in northeastern Iran. The remarkable occurrence of visceral leishmaniasis in asymptomatic sheepdogs reflects a health alert to conduct the surveillance and monitoring of susceptible individuals/reservoirs in the region.