1. Background
Globally, infectious diarrhea is the second common cause of mortality and morbidity in children aged below five years. It is also responsible for the annual death of 525,000 children (1, 2). Dehydration is mostly caused by the loss of an excessive amount of body fluids and electrolytes through prolonged diarrhea, which can lead to serious complications in young children and infants (3). Various types of bacteria, viruses, and parasites may cause prolonged or persistent diarrhea (4). Specifically, among young children in low-income countries, Enteropathogenic Escherichia coli (EPEC) is one of the most important causes of persistent diarrhea worldwide (5, 6). The EPEC pathogenesis depends on carrying the attaching-effacing (A/E) lesions, resulting in the destruction of brush-border microvilli, intimate attachment of bacteria to intestinal epithelial cells, and finally, formation of pedestal-like structures at the site of bacterial adherence (7).
All of the genes necessary for A/E lesion formation are located on a pathogenicity island called the locus of enterocyte effacement (LEE) that encodes the intimin adhesion, which is encoded by the eae gene and several effector molecules secreted by the type III system (8, 9). Moreover, EPEC contains a large plasmid, recognized as the EPEC adherence factor (EAF) plasmid with bfp encoding the type IV pilus (bundle-forming pilus [BFP]), which contributes to early localized adherence to epithelial cells and microcolony formation (10). Enteropathogenic Escherichia coli and Shiga-toxin producing E. coli (STEC) could produce A/E lesion by eaeA chromosomal gene, but shiga toxin encoding gene (stx-1, stx-2) only is present in STEC, which is used for distinguishing between these pathotypes.
In addition, the EPEC pathotype is divided into typical (tEPEC) and atypical (aEPEC) EPECs. However, just the tEPEC contains the EAF plasmid that encodes the bundle-forming pilus (BFP). The classification of EPEC isolates is based on the presence of EAF plasmid in typical EPEC (eae A+, bfp +, stx -), while the absence of this virulence factor in atypical EPEC (eae A+, bfp -, stx -) (5, 11, 12). In contrast to typical EPEC, which is accepted as a diarrhea pathogen, the pathogenic potential of a-EPEC is still controversial (13). Although many studies have reported a significant association between EPEC and diarrhea in children, the results of some of them indicated the presence of EPEC in asymptomatic cases (5, 14).
Atypical EPEC might possess potential virulence factors (e.g., efa1/lifA), which is outside the LEE and inside PAI O122 (15-17). The efa1/lifA gene has the product lymphostatin/Efa1, which inhibits the production of lymphokines and mitogen-activated proliferation of peripheral blood lymphocytes and gastrointestinal lymphocytes (18, 19). Normally, EPEC-induced diarrhea is a self-limiting disease and can be effectively treated with oral rehydration therapy. However, antimicrobial therapy might be necessary for severe persistent infections. It is notable that the use of antibiotics may be associated with complications, including drug toxicity and increased widespread antimicrobial resistance in patients (4, 20).
According to the literature, Enterobacteriaceae produce Extended-spectrum-β-lactamases (ESBL) enzymes that hydrolyze beta-lactam antibiotics. The prevalence of infections by these strains has been on the rise across the world. In addition, ESBL production is the main factor for the spread of MDR isolates and is classified into TEM, CTX, SHV, PER, and OXA types based on the sequence of amino acids. Currently, CTX-M group is the most frequently detected type of ESBL-related genes. Therefore, the detection of resistant isolates due to this mechanism is important within every area (21, 22).
2. Objectives
Considering this background, the present study aimed to assess the prevalence of atypical and typical EPEC among a collection of E. coli isolates obtained from children aged below 10 years applying PCR amplification of eae, bfp, and eaf genes in Ahvaz, Iran. In addition to determining the antibiotic susceptibility profiling and potential ESBL production of EPEC isolates, the researchers made efforts to the recognition of efa1/lifA gene as a virulence factor in atypical EPEC strains.
3. Methods
3.1. Bacterial Strains
Fecal samples of children below the age of ten years with diarrhea were collected during March 2015-February 2016 in two educational hospitals of Ahvaz, Iran. Samples were screened for E. coli strains using standard biochemical tests. Stool samples cultured on MacConkey agar and incubated at 37ºC for 24 hours. Identification of E. coli strains was performed based on the standard biochemical tests such as oxidase negative, catalase-positive, carbohydrate utilization on TSI agar, methyl red positive, Voges-Proskauer negative, indole positive, citrate negative, and urease negative (23).
3.2. PCR Methods
The genomic DNA was extracted from the bacterial culture using the boiling method as previously described (24). At least two colonies of EPEC isolates were suspended in one ml distilled water and incubated at 95ºC for 10 minutes. Then the suspension was centrifuged for 5 minutes at 1,000 rpm. Supernatants were transferred to new tubes and used for PCR reactions (25). The quality of extracted DNA was measured by biophotometry (Eppendorf, Hamburg, Germany) at OD260 and OD280 nm, and agarose gel electrophoresis, respectively. Primer sequences for the study assays eaeA, bfpA (26), stx1, stx2 (27), and efa1/lifA (this study) are shown in Table 1. The sequence of efa1/lifA primers was constructed using the oligo primer analysis software V. 7, and a suitable primer was selected on the basis of specificity and thermodynamic properties for good primer design. Primer specificity was checked using the basic local alignment search tool (BLAST).
| Primer | Primer: Sequences 5’ - 3’ | Product Size (bp) | Annealing (°C) | Reference |
|---|---|---|---|---|
| eaeA | 790 | 55 | 23 | |
| eaeA-F | CATTATGGAACGGCAGAGGT | |||
| eaeA-R | ATCTTCTGCGTACTGCGTTCA | |||
| bfpA | 326 | 60 | 23 | |
| bfpA-F | AATGGTGCTTGCGCTTGCTGC | |||
| bfpA-R | GCCGCTTTATCCAACCTGGTA | |||
| stx-1 | 614 | 56 | 24 | |
| stx-1-F | ACACTGGATGATCTCAGTGG | |||
| stx1-R | CTGAATCCCCCTCCATTATG | |||
| stx-2 | 779 | 56 | 24 | |
| stx2-F | CCATGACAACGGACAGCAGTT | |||
| stx2-R | CCTGTCAACTGAGCAGCACTTTG | |||
| efa1/lifa | 392 | 59 | This study | |
| efa1/lifa F | CATTGTCGTAGCAACCCTG | |||
| efa1/lifa R | GTGGCGAGAGGTAGAATCCG | |||
| blaCTX-M | 550 | 54 | 26 | |
| CTX-M-F | CGCTTTGCGATGTGCAG | |||
| CTX-M-R | ACCGCGATATCGTTGGT | |||
| blaTEM | 800 | 52 | 26 | |
| TEM-A | GAGTATTCAACATTTCCGTGTC | |||
| TEM-B | TAATCAGTGAGGCACCTATCTC | |||
| blaPER | 925 | 56 | 26 | |
| PER-F | AATTTGGGCTTAGGGCAGAA | |||
| PER-R | ATGAATGTCATTATAAAAGC |
The Primer Sequences of the Research
The eaeA and bfpA genes as control strains were prepared from the National E. coli Reference Laboratory (NERL), Pasteur Institute of Iran. In addition, E. coli ATCC 43894 and EPEC O127:H6 clinical isolate were used to (Stx-1 and Stx-2) and (efa1/lifA) genes as control strains, respectively. Escherichia coli isolates were examined for the presence of the eaeA, bfpA, and efa1/lifA virulence genes by PCR. The presence of stx-1 and stx-2 virulence factors were determined by multiplex PCR. PCR reaction contained 2.5 µL 10X PCR buffer, 1 mM mgcl2, 1 mM concentration of each deoxynucleoside triphosphate, 0.5 U of Taq DNA polymerase, 1 µL of primers 10 ng primer/µL, 5 µL of DNA template, and sterile distilled water to a total reaction volume of 25 µL.
Furthermore, a thermal cycler was applied to carry out the amplification conditions (Eppendorf Mastercycler, Germany). Amplification conditions for PCR were as follows: Initial denaturation at 94ºC for 3 minutes; 35 cycles of 94ºC for 45 seconds, annealing at 52 - 60ºC for 45 seconds, extension at 72ºC for 1 minute, and a final extension at 72ºC for 5 minutes. Primer sequences and PCR conditions are listed in Table 1. PCR products were loaded on a 1.5% (w:v) agarose gel with 0.5 μg/mL and visualized using ultraviolet light after staining with ethidium bromide (CinnaGen Co., Tehran, Iran). Samples were classified as containing EPEC in case of being eaeA-positive and stx-negative, and then as t-EPEC (bfp-positive) or a-EPEC (bfp-negative). A-EPEC samples were further classified as efa1/lifA-positive or negative.
3.3. Enteropathogenic Escherichia coli Serogrouping
Serogrouping with polyvalent antisera (Baharafshan Institute of Research and Development, Tehran, Iran) was performed to confirm EPEC strains according to the manufacturer’s instructions. The polyvalent antisera are composed of four separated pool sera, able to react with the following serogroups: polyvalent 1 (O26, O55, and O111), polyvalent 2 (O86 and O127), polyvalent 3 (O44, O125, and O128), and polyvalent 4 (O20 and O114).
3.4. Antibiotic Resistance Profiling of Escherichia coli Strains
In this research, a disk diffusion test was performed to determine the antibiotic resistance/susceptibility profiles of EPEC strains (28). Ten commercial antibiotic discs (Mast Diagnostics, Merseyside, UK) were performed for testing the susceptibility, including Amikacin (AN: 30 µg), Ampicillin (AM: 30 µg), gentamicin (GEN: 30 µg), ceftriaxone (CRO: 30 µg), ceftazidime (CAZ: 30 µg), cefotaxime (CTX: 30 µg), cefoxitin (FOX: 30 µg), imipenem (IPM: 10 µg), meropenem (MEM: 10 µg), ciprofloxacin (CIP: 5 µg), co-trimoxazole (SXT: 25 µg), tetracycline (TE: 30 µg) and colistin (CO, 10 µg). Moreover, the antibiotic susceptibility tests were performed using E. coli ATCC 25922 strain as a control.
3.5. Phenotypic and Genotypic Detection of ESBL Producers
The resistant isolates to at least one of the third-generation cephalosporins (i.e., ceftazidime and cefotaxime) were screened to detect ESBL producers by combined disk test (CDT). In this respect, ESBL was detected by a double-disk-diffusion test using cefotaxime, ceftazidime discs (30 mg) with and without of clavulanic acid (10 mg) according to CLSI (28). Isolates that increased by ≥ 5 mm in the zone diameter in the presence of clavulanate disk was confirmed as ESBL producers. Escherichia coli ATCC25922 and Klebsiella pneumoniae ATCC700603 were used as negative and positive controls for ESBL production, respectively. PCR was performed for the detection of blaCTX-M, blaTEM, and blaPER genes with specific primers as described previously, with the exception of the annealing temperatures (29) (Table 1). In addition, K. pneumonia strain 7881 and P. aeruginosa KOAS strains were used as the positive controls.
3.6. Statistical Analysis
Data were analyzed with SPSS version 16 using the chi-square test. Moreover, a P value of less than 0.05 was considered statistically significant.
4. Results
In the current study, 251 E. coli isolates were identified from children below the age of 10 years with acute diarrhea using culture and biochemical methods. In total, 64.70% of the subjects were male, and the rest (35.30%) were female, which was indicative of the higher number of males in the research. Most EPEC isolates (42.1%) were isolated from patients in spring, followed by summer (26.3%).
4.1. Enteropathogenic Escherichia coli Isolation
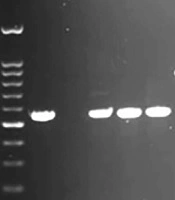
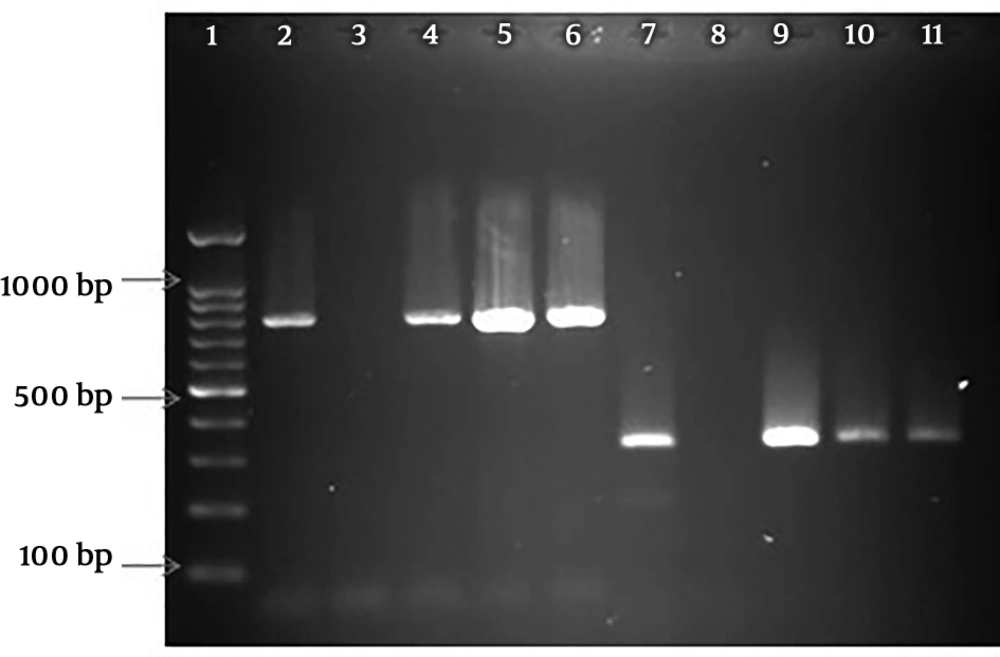
The amplified PCR products of EPEC virulence for eaeA and bfpA genes on 1.5% agarose gel are shown in Figure 1. This figure also reflects the multiplex PCR for stx-1 and stx-2 genes. Among the 251 E. coli isolates, 17 (6.78%) were identified as EPEC. All EPEC strains were positive for the eaeA gene and negative for stx-1 and stx-2 genes. In addition, typical EPEC [eaeA + bfpA] and atypical EPEC (eaeA) were found in 6 (35.3%) and 11 (64.7%) of EPEC isolates, respectively. In addition, the efa1/lifA gene was not observed in any of the a-EPEC samples (Data not shown).
PCR amplified products of virulence genes. Lane 1: 100 bp molecular weight marker; lane 2: control positive of eaeA (790bp); lane 3: control negative of eaeA; lane 4, 5, and 6: eaeA positive of clinical isolates; lane 7: control positive of bfpA (326 bp); lane 8: control negative of bfpA; lane 9, 10, 11: bfpA positive of clinical isolates
4.2. Serogrouping
The results of O serogrouping revealed that only 8 (4.7%) isolates of the 251 E. coli isolates were typeable with EPEC polyvalent antisera. These eight isolates belonged to eight serogroups, namely O26, O55, O111 (5 isolates), O44, O125, O128 (2 isolates), and O86, O127 (One isolate).
4.3. Antibiotic Susceptibilities of EPEC Isolates
The antibiotic susceptibility testing results of the confirmed EPEC strains are presented in Table 2. All of the isolates were susceptible to colistin, imipenem, and meropenem. In addition, the highest level resistance in these isolates was found against ampicillin (100%), followed by resistance to ceftriaxone and co-trimoxazole (76.47%). It is notable that children aged ≥ 2 years showed the highest resistance (72%) to tetracycline (P < 0.05), while only 28% of children aged < 2 years were resistant to this antibiotic. Antibiotic resistance patterns of EPEC isolates are shown in Table 3.
| Antibiotics | Resistant (%) | Intermediate (%) | Sensitive (%) |
|---|---|---|---|
| Amikacin | 1 (5.88) | 2 (11.76) | 14 (82.35) |
| Ampicillin | 17 (100) | 0 (0) | 0 (0) |
| Ceftazidime | 6 (35.29) | 4 (23.52) | 7 (41.17) |
| Cefotaxime | 11 (64.7) | 0 (0) | 6 (35.29) |
| Cefoxitin | 4 (23.52) | 0 (0) | 13 (76.47) |
| Ceftriaxone | 13 (76.47) | 0 (0) | 4 (23.52) |
| Ciprofloxacin | 6 (35.29) | 2 (11.76) | 9 (52.94) |
| Colistin | 0 (0) | 0 (0) | 17 (100) |
| Co-trimoxazole | 13(76.47) | 0 (0) | 4 (23.52) |
| Gentamicin | 2 (11.76) | 0 (0) | 15 (88.23) |
| Imipenem | 0 (0) | 0 (0) | 17 (100) |
| Meropenem | 0 (0) | 0 (0) | 17 (100) |
| Tetracycline | 6 (35.29) | 0 (0) | 11 (64.7) |
The Antibiotic Susceptibility Testing Results of Enteropathogenic Escherichia coli Isolates
| Resistance Pattern | Phenotypic Resistance | Number of Resistant Isolates (%) |
|---|---|---|
| 1 | AMP | 2 (11.7) |
| 2 | AMP-CRO | 1 (5.8) |
| 3 | AMP-TET | 1 (5.8) |
| 4 | AMP-FOX-SXT | 1 (5.8) |
| 5 | AMP-CRO-SXT-TET | 1 (5.8) |
| 6 | AMP-CRO-CTX-GEN | 1 (5.8) |
| 7 | AMP-CAZ-CRO-CTX-SXT | 2 (11.7) |
| 8 | AMP-CIP-CRO-CTX-SXT | 1 (5.8) |
| 9 | AMP-CAZ-CIP-CRO-CTX-SXT | 1 (5.8) |
| 10 | AMP-CAZ-CRO-CTX-SXT-TET | 2 (11.7) |
| 11 | AMP-CAZ-CRO-CTX-FOX-SXT | 1 (5.8) |
| 12 | AMP-AN-CAZ-CIP-CRO-CTX-SXT | 1 (5.8) |
| 13 | AMP-CAZ-CIP-CRO-CTX-FOX-SXT-TET | 2 (11.7) |
Antibiotic Resistance Phenotypic Profiles of EPEC Isolates
4.4. Phenotypic ESBL Detection
Fourteen isolates resistant to cefotaxime and ceftazidime were assessed using the phenotypic combination disk test (CDT) for evaluation of ESBL production. In total, 13 (76.47%) of these isolates were found to be ESBL producers.
4.5. Detection of β-lactamase Genes
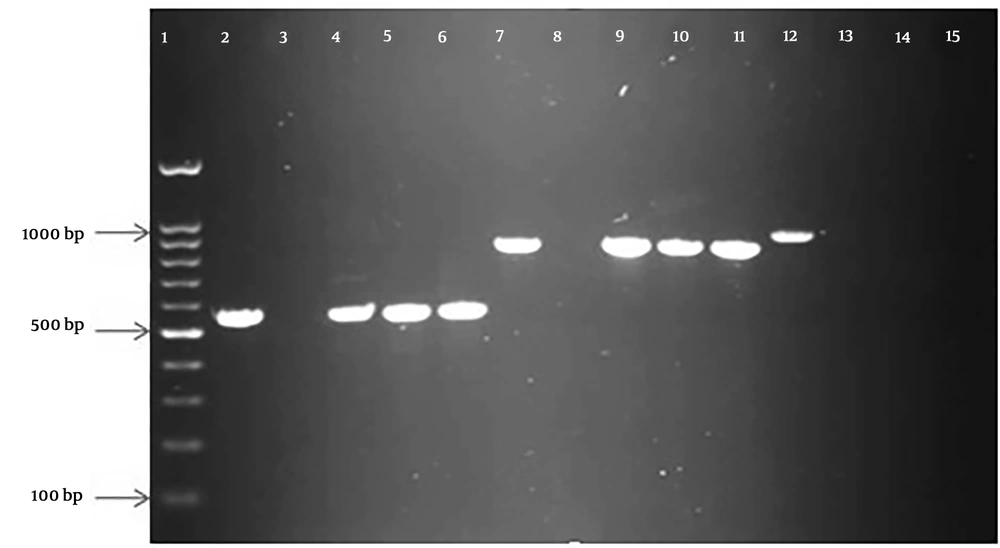
In this research, PCR was performed for all EPEC strains, where 12 (70.58%) out of 17 ESBL producers carried the blaCTX-M gene. Moreover, the rate of the blaTEM gene among these isolates was 10 (58.82%). In the positive isolates for these genes, five typic and seven atypic for the blaCTX-Mgene and five typic and five atypic for blaTEM gene were obtained. Interestingly, 𝑏𝑙𝑎PER genes were not detected in all isolates (Figure 2). According to the results, no significant relationship was observed between the frequencies of typical or atypical EPEC isolates and factors of age, gender, or antimicrobial resistance patterns (except for tetracycline). Moreover, no significant association was found between antibiotic resistance and variables of gender and age. However, children below two years were more susceptible to tetracycline. In total, susceptibility to tetracycline was found in 72% and 28% of children aged below two years and above this age, respectively. All of the children under the age of years were susceptible to this antibiotic, resistance to which was presented in children with mean age above two years (P < 0.05). On the other hand, no significant correlation was detected between drug resistance and the presence of bfpA gene. The detailed characteristics of all EPEC isolates, including typic and atypic EPEC, and phenotypic and genotypic ESBL production are summarized in Table 4.
PCR amplified products of β-lactamase genes in the study. Lane 1: 100 bp molecular weight marker; lane 2, 3, 4, 5, and 6: control positive, control negative, and three clinical isolates of blaCTX-M (550 bp); lane 7, 8, 9, 10, and 11: control positive, control negative, and three clinical isolates of blaTEM (800 bp); lane 12, 13, 14, and 15: control positive, control negative, and two clinical isolates of blaPER(925 bp).
| EPEC No. | Typic/Atypic Isolates | ESBL Phenotype | ESBL Gene Pattern |
|---|---|---|---|
| 1 | Atypic | Neg | None |
| 2 | Typic | Pos | TEM, CTX-M |
| 3 | Atypic | Pos | TEM, CTX-M |
| 4 | Atypic | Pos | CTX-M |
| 5 | Atypic | Pos | CTX-M |
| 6 | Atypic | Pos | TEM |
| 7 | Atypic | Neg | None |
| 8 | Atypic | Pos | TEM, CTX-M |
| 9 | Atypic | Pos | TEM, CTX-M |
| 10 | Typic | Neg | None |
| 11 | Atypic | Pos | CTX-M |
| 12 | Atypic | Neg | CTX-M |
| 13 | Typic | Pos | TEM, CTX-M |
| 14 | Typic | Pos | TEM, CTX-M |
| 15 | Typic | Pos | TEM, CTX-M |
| 16 | Atypic | Pos | TEM |
| 17 | Typic | Pos | TEM, CTX-M |
The Detailed Results Typic/Atypic Isolates, ESBL Phenotype and Genotype Patterns in EPEC Isolates
5. Discussion
According to the literature, EPEC is a significant cause of acute diarrhea, especially in developing countries. Owing to their high prevalence of this type of E. coli in both community and hospital settings, it is responsible for approximately 11% of all diarrhea mortalities in children aged below five years in the world (1, 2). Moreover, severe malabsorption of nutrients might be caused by EPEC, helping the nutritional aggravation and the persistence of diarrhea (5). In the present study, the results of PCR detection of eaeA gene were indicative of 6.78% frequency of EPEC in the E. coli isolates, which was higher, compared to the prevalence rate mentioned in two reports by Nakhjavani (5.6%), Asadi Karam (5.3%) and lower than report by Moshtagian (21.5%) in Iran (30-32), and also higher from some neighboring countries, including Iraq (3.4%) and Turkey (2.05%) (33, 34). This rate was also lower, compared to the reports from Kuwait (8.4%) and Pakistan (35, 36). The results of previous studies propose that differences in the type of samples, method for sampling, geographical area, antibiotic prescription are important criteria in epidemiology in children with diarrhea, which leads to different data between these investigations (37).
The presence of stx and/or eaeA genes can distinguish Shiga toxin-producing E. coli strains (STEC) from EPEC strains (38). Similar to numerous studies (33, 39), none of the E. coli isolates were positive for the stx gene in the present study, which resulted in a lack of their characterization as STEC. In clinical laboratories, serogrouping with O-type antisera is still a useful diagnostic method to determine a limited number of EPEC serogroups (40). In the current study, nearly half of EPEC strains were not typeable with diagnostic antisera and often belonged to the atypical group. In this regard, our findings are consistent with other studies (41), which demonstrated that serogrouping is a tedious, laborious, and time-consuming process and fails to identify some of the EPEC strains. Therefore, PCR methods can be a reliable, fast, and sensitive alternative applied to identify the EPEC strains that belong to serogroups not detected by commercially available antisera (39, 40).
Moreover, our findings revealed the role of EPEC strains, which belong to serogroups O26, 055, and O111, in the majority of EPEC diarrhea in the southwest of Iran, Ahvaz. In accordance with the present study, O55, O111, and O26 serogroups were responsible for 54.5% of diarrhea in children of Iraq (33). Meanwhile, the most prevalent serogroups in Iran are O127 and O128, respectively (26, 41). Depending on the presence or absence of the EPEC adherence factor plasmid (pEAF), EPEC may be subdivided into typical (tEPEC; eaeA+ and bfpA+) or atypical (aEPEC; eaeA+ bfpA–) EPEC (12). In the current research, approximately 65% of the 17 tested EPEC isolates were subdivided as atypical EPEC strains. These findings are in congruence with other reports, indicating the high prevalence of EPEC strains among young children in developing countries (5, 42, 43).
The exact mechanisms of aEPEC-induced diarrhea are still not completely understood (43, 44). Recently, Afset et al. detected a significant association between the prevalence of diarrhea and the presence of the O island 122 (OI-122), including efa1/lifA and several other genes (45). Moreover, there is the possibility of a correlation between the pathogenesis of aEPEC and its serogroups. In the present study, efa1/lifA genes were found in none of the aEPEC serogroups. In this respect, our findings are in line with the idea that EPEC strains from different serogroups may contain various pathogenic genes. EPEC diarrhea is often mild and self-limiting and effectively treated with oral rehydration therapy. Meanwhile, persistent infections may require the use of antimicrobial treatment (4, 20).
In the current study, antibiotic susceptibility testing was performed on the EPEC isolates. According to the results, the highest resistance rates were related to ampicillin (100%), ceftriaxone (76.5%), cotrimoxazole (70.6%), cefotaxime (64.7), and ceftazidime (52.9%), respectively. However, all EPEC isolates were sensitive to colistin, meropenem, and imipenem. Several studies have reported various levels of antibiotic resistance in EPEC isolates from developed and developing countries. Diversity in the time and region of these investigations might have led to these conflicting results. Compared to the present research, previous studies in Iran have reported a low prevalence of resistance to ampicillin, cotrimoxazole, cefotaxime, and ceftazidime (30, 41). In a study in India, a lower percentage of drug resistance to cotrimoxazole (35.49%), ceftriaxone (32.20%), and ciprofloxacin (25.42%) was described. In addition, while resistant to meropenem was reported at (25.42%), all EPEC isolates were susceptible to imipenem, which is in congruence with our findings (42). In Iraq, the high prevalence of resistance was related to ampicillin (97.4%), cotrimoxazole (82%), cefotaxime (89.7%), and ceftazidime (79.5%) (46).
Resistance to carbapenems is of significant importance in the treatment of patients infected with gram-negative bacteria. The carbapenemase enzymes can cause pan-drug resistant (PDR) strains since they regularly transport on plasmids in combination with other resistance genes, especially ESBL genes. According to the results of the present research, EPEC strains are highly susceptible to carbapenem antibiotics and have not yet gained the carbapenemase genes. Therefore, the clinicians should consider this issue in prescribing antibiotics to patients in this area. Conflicting reports have been reported by researchers in Iran, including Memariani et al., who showed a lack of ability to achieve any resistance to imipenem. On the other hand, Nakhjavani et al. marked that resistance to imipenem in the EPEC strain was 26% (30, 41). In a study in India, the prevalence of imipenem and meropenem resistance was 15% and 2.5%, respectively (47). Moreover, no resistance to imipenem was reported in Kuwait (38).
In susceptibility tests, multidrug-resistant strain (MDR) is used for isolates with resistance to three or more antibiotics. A total of 13 (76.47%) EPEC isolates were MDR, 53.3% of which were atypical, and 33.3% were typical strains. Some studies have reported fewer number of these strains. For instance, 45.83% of MDR strains were reported in India (47). A combined disk test (CDT) is performed for all the strains that are resistant to cephalosporine antibiotics, such as cefotaxime, ceftazidime, cepdotoxime, and ceftriaxone. Among these strains, a positive result was obtained in 13 (76.47%) of EPEC strains by CDT in the current investigation. Among the evaluated ESBL producer isolates, the prevalence of blaCTX-M and blaTEM was 12 (70.5%) and 10 (58.8%) in genotypic detection of beta-lactamase genes, respectively. Another research reported 21% ESBL-positive by CDT, 88.8% blaCTX-M, and 19% blaTEM found in these strains by genotypic test in Iran (41). Other data have revealed 45.83% positive isolates in CDT test. Moreover, blaTEM and blaCTX-M were displayed in 35.5% and 19.5% of EPEC isolates from India (47). In the case of phenotypic and genotypic ESBL tests, few studies have been carried out, yielding conflicting results.
5.1. Conclusions
In the current study, EPEC isolates had high resistance to third-generation cephalosporins and fluoroquinolones. Carbapenems are highly effective drugs for the treatment of such infections. The importance of MDR strains-producing β-lactamase enzymes is high-level drug resistance to main antibiotics, such as third-generation cephalosporins, fluoroquinolones. Regarding the frequency of β-lactamase genes in EPEC strains, treatment of these multidrug-resistant organisms remains a scientific concern; therefore, it is recommended that a sufficient amount of antibiotics should be carefully prescribed for the treatment of these bacteria.