1. Context
Coronaviruses (CoVs) belong to the group of RNA viruses that cause respiratory and gastrointestinal infections in humans and animals. Scientists prove that coronaviruses existed before approximately 8,000 BC (1). Since bats and birds are among the main hosts of coronaviruses, it is recognized that they are mainly involved in their evolution and spread (2). Human history is accompanied by a long history of CoV mutation and transmission among animal and human (animal-animal-human) hosts. This is documented by reports of diseases caused by CoV in cattle, Equidae, dogs, and humans (3). First reports of human coronaviruses come from the 1960s (4). Two pathogens, namely HCoV-229E and HCoV-OC43 have been described, which were then isolated and characterized as responsible for colds and self-limiting upper respiratory tract infections in people without aggravating diseases (5). Coronaviruses have been responsible for mild respiratory and gastrointestinal infections for years, and only the last 18 years reveal their acute, highly contagious, and epidemic nature.
At the end of 2002, an outbreak of disease caused by the previously unknown, highly contagious coronavirus species, severe acute respiratory syndrome (SARS-CoV) occurs in the south of China. The disease syndrome caused by this pathogen is severe acute respiratory failure, which is characterized by lung tissue damage. The SARS-CoV epidemic has spread to 37 countries (6). As a result, 8,273 cases of infection were discovered, 775 were fatal (7-9). SARS-CoV mortality was 17% (10). The end of the epidemic occurred in July 2003, when the World Health Organization (WHO) announced the eradication of the SARS virus. In 2012, new coronavirus Middle East respiratory syndrome (MERS-CoV) appeared. The first outbreak occurred in Saudi Arabia, while the course was a form of a severe, often fatal respiratory disease. In December 2019, another epidemic broke out in Wuhan, China, caused by a new coronavirus called SARS-CoV-2. It causes the highly contagious COVID-19 in which common symptoms are fever, cough, shortness of breath, chest pain, and severe breathing difficulties (11). The epidemic is rapidly expanding to other countries and continents, and at 11/03/2020, WHO announces a pandemic caused by SARS-CoV-2.
This review summarizes current information on the emergence, origin, diversity, and common characteristics, as well as the epidemiology of the above three highly contagious coronaviruses.
2. Structure and Systematics of Coronaviruses
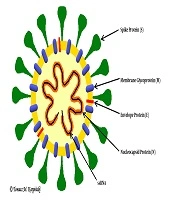
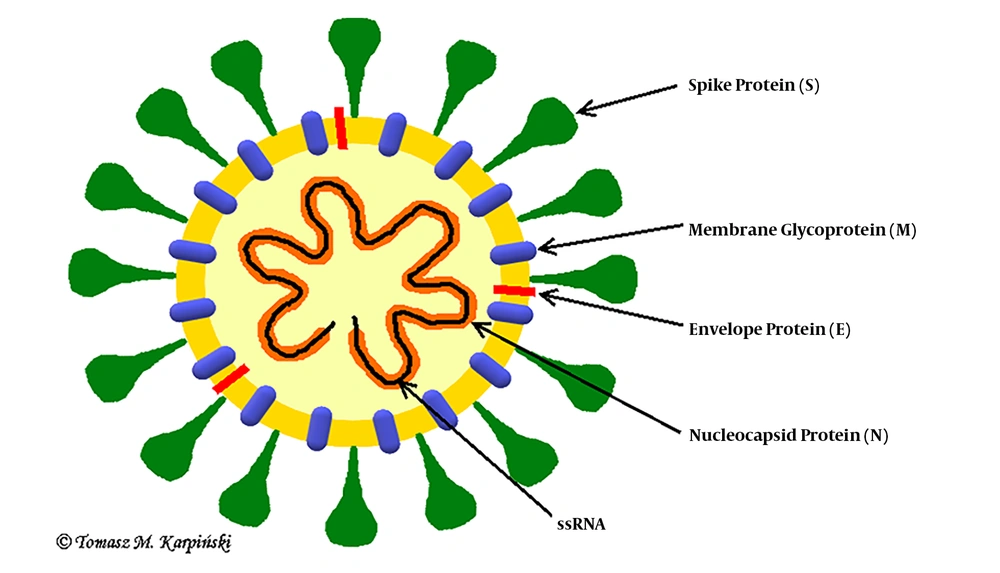
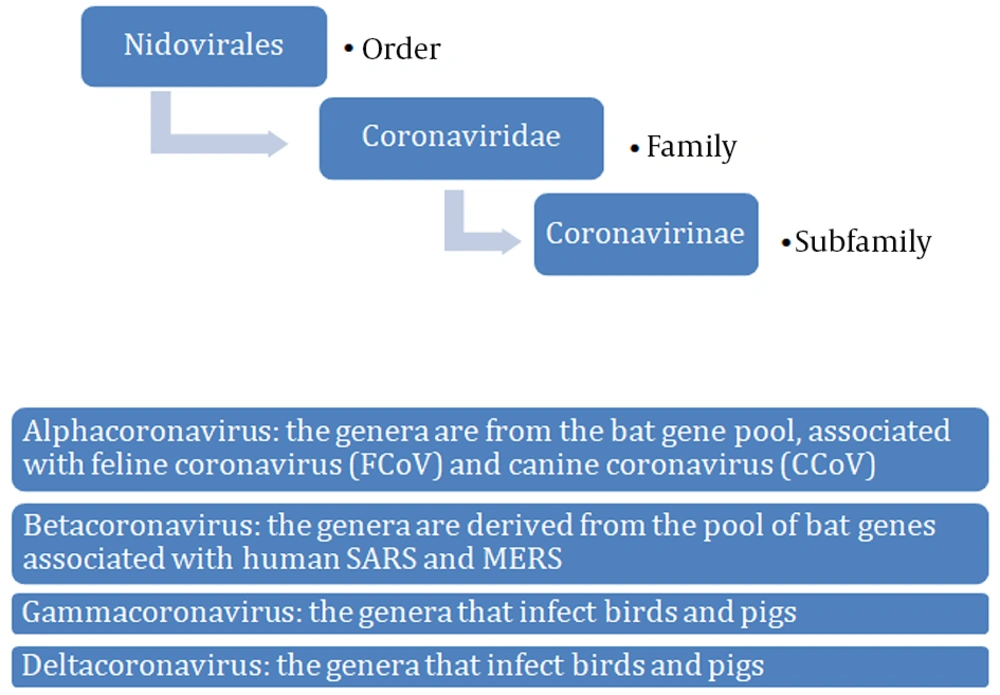
Coronaviruses are single-stranded RNA viruses (Figure 1) with positive polarity and helical symmetry of the nucleocapsid (12). Coronaviruses have the largest genome among RNA viruses (~30,000 nucleotides). The appearance resembles a crown due to the presence of spiky glycoproteins on the envelope. RNA molecule ranges from 26 - 32 kb and contains at least six open reading frames (ORFs). The first ORF (ORF1a/b) comprises about two-thirds of the genome and encodes a replicase protein (13). The remaining one-third of the genome encodes four structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins. Some coronaviruses encode hemagglutinin esterase (HE), which may be involved in virus entry or exit (14). SARS-CoV, MERS-CoV, and SARS-CoV-2 belong to the Coronaviridae family and Coronavirinae subfamily (Figure 2) (15). All known and most relevant coronaviruses infecting humans belong to the groups of alpha and beta viruses. Both alpha- and beta-coronaviruses infect bats, and they can also infect other species, including humans, camels, and rabbits. The zoonotic coronavirus reservoir allows widespread in the environment, and it is a source of infection for other susceptible species. One reservoir host may be infected several times (3, 16).
3. Epidemiology
3.1. SARS-CoV
The first cases of the novel etiological factor infections, causing acute atypical highly contagious pneumonia, took place in Chinese Province Guangdong in November 2002.In February 2003, in China, 305 cases were confirmed, including 5 deaths (17). In March 2003, the WHO declared a new disease, namely SARS (7). The outbreak of SARS occurred in Hong Kong, Vietnam, Singapore, Canada, and the USA (6). The case fatality rate was 9.6 (7, 10).
3.2. MERS-CoV
MERS-CoV was first isolated in June 2012 in Jeddah, Saudi Arabia. MERS-CoV outbreaks were noted in 26 countries, mainly in Saudi Arabia (1,037 cases), South Korea (185), and the United Arab Emirates (76). The total number of MERS cases in Europe was 15 (including 7 deaths). Cases have been detected in Great Britain, Germany, France, and the Netherlands. Since 07/07/2015, a total of 1,368 cases have been identified and laboratory-confirmed, including 487 deaths. MERS has a high mortality rate, which reached 36%, especially in elderly patients and patients with chronic diseases (18).
3.3. SARS-CoV-2
The first case of infection with a new type of coronavirus occurred at the Huanan Seafood Market in Wuhan, China, in December 2019 (19). On the fish and seafood market in Wuhan, the meat of various animal species, including pigs, sheep, camels, foxes, badgers, and multiple reptiles has been sold (20). On 31/12/2019, a group of acute respiratory diseases was reported to the WHO Country Office in China. On 07/01/2020, a new virus was isolated, and a new type of coronavirus was confirmed, which was initially named as a novel coronavirus (nCoV) (21). Since 13/01/2020, infections in other countries began to emerge, including Thailand, Japan, and South Korea. At the same time, fatalities began to be registered as a result of bilateral pneumonia of acute course, and SARS-CoV-2 etiology was recorded (11). On 04/03/2020, the first case of infection in Poland was confirmed. On 11/03/2020, the WHO declared COVID-19 disease caused by SARS-CoV-2 a pandemic. The number of confirmed infections in the world as according to the WHO, exceeded 7 million, while the number of deaths exceeds 400,000 so far. The largest outbreaks are concentrated in Europe, the United States, and Brazil.
4. Dissemination
Natural reservoir hosts of the viruses are bats. Bats (alpha and beta) and birds (gamma and delta) are considered to be the main hosts of evolution and the spread of coronaviruses. Before SARS-CoV, MERS-CoV, and SARS-CoV-2 become pathogenic to humans, a so-called species breakthrough has to occur twice: first, between bats (22, 23) and other mammals (vectors); next, between mammals and a human. In the case of SARS, animal vectors were palm civets, in case of MERS -camels, and in the case of SARS-CoV-2- possibly snakes (24-26).
4.1. SARS-CoV
Scientists from Hong Kong have detected the presence of the SARS virus in the organisms of Tibetan civet cats (civets). Chinese researchers have isolated anti-SARS-CoV antibodies from the serum of civet vendors. The virus found in civets proved to be almost identical in terms of the structure of genetic material to the SARS virus responsible for the pandemic (27). The main route of transmission is considered to be the short distance droplet route of infection and direct contact with a sick person. The airways secretion of the ill person contains the highest number of copies of the virus (9). Furthermore, it is possible to transmit the virus on objects contaminated with body fluids and secretions of sick people. The SARS virus can survive for up to 48 hours in drying excretions or systemic fluids. Another route of transmission seems to be the air route, e.g., through contaminated air-conditioning systems, as it was observed in Hong Kong, where more than 300 people became ill in a short time (8, 9).
4.2. MERS-CoV
The first cases in humans have been reported on the Arabian Peninsula as a result of direct contact with infected monotropic camels (dromedaries) or indirectly, with their excretions and secretions (feces, urine, milk, respiratory tract secretions) (28). Consumption of unpasteurized camel milk or improperly heat-treated meat is a source of infection (29). Epidemiological and genetic studies have confirmed that MERS-CoV is a zoonotic virus. The prevalence of specific antibodies in camel herds on the Arabian Peninsula and North Africa has been demonstrated, which proves the circulation of MERS-CoV in these animals for decades (30). Secondary infections are droplet-transmitted from human to human (an epidemic in South Korea in 2015).
5. Clinical Symptoms
5.1. SARS-CoV
The period of incubation is 2 - 10 days. In the case of SARS, predominant symptoms are fever > 38°C, chills, headaches, dizziness, muscle aches, sore throat, dry cough. Shortness of breath, nausea, vomiting, diarrhea have been observed less often. The course of the disease can vary significantly from a non-symptomatic form to severe respiratory failure in about 20% of patients, usually resulting in death. An essential clinical feature of SARS is the dynamics of ARDS development. This complication occurred in about 16% of all patients with SARS, and when it happened, it was associated with a 50% mortality rate (31). Deterioration of a patient’s clinical condition was observed between the 7th and the 10th day from the first symptoms (32). The total mortality rate at the outbreak was estimated at 9.6% (33). In the profile of laboratory tests, leukopenia with lymphopenia, thrombocytopenia, increased activity of lactate dehydrogenase, creatinine kinase, aspartic aminotransferase were present (34).
5.2. MERS-CoV
The incubation period of MERS lasts 2 to 10 days (mean 5 - 6 days); death occurs approximately after 11.5 days (35). MERS-CoV infections predominated in men, more than half of the cases involved people over 50 years old (36). The most frequent clinical symptoms are fever > 38°C, cough, headaches, muscle, joint pains, breathing difficulties, and shortness of breath. Abdominal pains, vomiting, and diarrhea have been observed less often (37). In the course of infection, severe pneumonia, acute respiratory failure, septic shock and multi-organ failure leading to death may develop, especially in elderly people (> 65 years) and patients with chronic disease (cardiovascular failure, respiratory diseases, kidney failure, diabetes, acquired or congenital immune disorders, cancer, etc.) (34). Simultaneously, a mild or asymptomatic course of infection in some patients has been observed (38).
5.3. SARS-CoV-2
The incubation period is 4 - 7 days (mean 5 days) (2). In the course of the disease, most patients develop dyspnea and pneumonia (19). Various progression of the disease has been observed, from mild cases to respiratory failure requiring mechanical ventilation in the intensive care unit (11). The most frequent complications are ARDS, acute myocardial damage, and secondary bacterial infections. Laboratory tests reveal the following deviations: leukopenia with lymphopenia, thrombocytopenia, high values of C-reactive proteins (CRP), and low values of procalcitonin. CT scans of the thorax show inflammatory changes in pulmonary tissue (39). The course of the infection depends on the age of the patient and the coexisting diseases.
6. Patomechanism
6.1. SARS-CoV
SARS-CoV enters host cells through interaction of protein S with the human angiotensin-converting enzyme-2 (ACE2) (39). Mainly these are ciliary epithelial cells of the respiratory tract and nasopharynx (40). The mechanisms of causing the disease are the direct lytic action of the virus on the host cells and the response of the host immune system to infection. Immunological response to the viral invasion and replication is a combination of early congenital and subsequent adaptive responses (41). In vitro tests on animal models have shown that replication of coronavirus in a host cell leads to cell necrosis, lysis, apoptosis, or cell fusion with the production of syncytium (42, 43). The acute inflammatory response in humans causes spilled damage to the alveoli, and giant cell infiltrates into the lung tissue (44). Also, acute hepatitis caused by viral damage and hematological disorders results from the direct viral mechanisms of the immune system reactions and not from the immediate action of SARS-CoV (45). Protein S may be the main factor determining the severity of the clinical disease due to its role in virus entry, pathogenesis, antiviral response, virulence, and cellular and species-specific tropism (46).
6.2. MERS-CoV
The respiratory tract is the entry of infection for MERS-CoV. MERS-CoV shows strong tropism to the cells of the unoccupied epithelium, which is an unusual feature among viruses attacking the respiratory system, as most of them infect the ciliary epithelium of the respiratory system. Viral glycoprotein S attacks the cellular receptor dipeptidylptyptidase 4 (DPP4), and leads to membrane fusion (47). The mechanism of pathogenic activity of MERS-CoV involves, among others, avoiding the mechanisms of the natural antiviral immune response (48).
6.3. SARS-CoV-2
It has been shown that SARS-CoV-2 uses an ACE2 and TMPRSS2 serine protease, which promotes entry into the host cell. TMPRSS2 activity is essential for the spread of the virus and pathogenesis in the infected host. Since the input to the host cell depends on the receptor, i.e., ACE2 and TMPRSS2 serine protease, it can be blocked by a clinically proven inhibitor of this cellular serine protease TMPRSS2. Moreover, studies show that the antibody response against SARS-CoV could at least partially protect from SARS-CoV-2 infection. These results show possibilities for SARS-CoV-2 therapy (49).
7. Diagnosis
7.1. SARS-CoV
The WHO has developed a definition of SARS that helps to provide the correct diagnosis. SARS should be suspected in a patient who had a fever (> 38°C) and coughing or breathing difficulties, and who, in 10 days preceding the onset of these symptoms, contacted a person likely to be infected with SARS, or who within 10 days prior to the onset of the symptoms, traveled to or resided in the areas where the spread of SARS was demonstrated. The test materials are respiratory tract secretions, urine, feces. The presence of genetic material of the virus is detected with molecular methods (50).
7.2. MERS-CoV
The WHO, the Centers for Disease Control and Prevention (CDC), and the Ministry of Health of Saudi Arabia have developed a definition of MERS that helps to make the correct diagnosis. Patients with fever and pneumonia or acute respiratory failure who are suspected of being infected with MERS-CoV should have a confirmed in their medical history stay in the Middle East. The stay should end no earlier than 14 days before the onset of the symptoms or a proven contact with travelers returning from those regions. Test material consists of bronchial tree secretion, bronchoalveolar lavage (BAL), taken during bronchoscopy (51), swabs from nose and throat, larynx, serum, and feces. The diagnosis of the disease should be confirmed by molecular biology (PCR) methods (52). The WHO recommends the immunofluorescence test for the serological diagnosis of infection primarily. The diagnosis shall be established on the basis of history, clinical picture and results of additional examinations (multifocal or bilateral exudative lesions in lung X-ray, leukopenia, lymphopenia, thrombocytopenia, high concentrations of creatinine, lactate dehydrogenase [LDH], alanine and aspartate transaminases [ALT and AST, respectively]) (53).
7.3. SARS-CoV-2
The test materials are nasopharyngeal swabs, samples from the lower respiratory tract, bubble and bronchial washings (BAL), bronchoaspirates, which have a higher diagnostic value than samples from the upper respiratory tract (e.g., nasopharyngeal swabs). The golden standard in diagnosing and confirming SARS-CoV-2 remains RT-PCR (54).
8. Treatment
8.1. SARS-CoV
There are no specific drugs for the treatment of SARS-CoV. The International SARS Treatment Study Group has not confirmed the effectiveness of ribavirin in combination with glucocorticosteroids, which was the most frequent treatment. Patients with respiratory failure require treatment in an intensive care unit and assisted breathing. Further studies indicate that lopinavir/ritonavir (protease inhibitors) reduce the risk of acute respiratory failure and death from SARS infection (55). It was demonstrated that IFN-β and IFN-γ can synergistically inhibit SARS-CoV replication in vitro (56). Antibody-containing plasma of convalescents is clinically useful in the treatment of SARS-CoV (57).
8.2. MERS-CoV
There are no specific drugs for the treatment of MERS-CoV infections. Oseltamivir, as well as glucocorticosteroids, were applied. For inhibition of replication are recommended: interferon α, interferon β, lopinavir, ritonavir, ribavirin, cyclosporine, and virus-cell receptor blockers (DPP4, also called CD26). No vaccine has been developed either (58). Patients with respiratory failure require maintenance treatment in an intensive care unit.
8.3. SARS-CoV-2
There is no effective treatment or vaccination against COVID-19 at present. Oseltamivir, antibiotics, and glucocorticosteroids are used empirically. Supportive symptomatic treatment is used, including oxygen therapy and mechanical ventilation. The recommendation of the Chinese Health Commission is the use of IFN-α and lopinavir/ritonavir as drugs (59). Lopinavir/ritonavir (protease inhibitors) has shown proven effectiveness in reducing the risk of acute respiratory failure or death in the case of SARS infection (56).
9. Discussion
In the view of the enormous public health threat posed by the recent coronavirus epidemics of SARS, MERS and the current pandemic caused by SARS-CoV-2, it is crucial to get to know biology, epidemiology, pathogenesis, diagnostics and clinical picture, in order to seek effective ways of treatment and prevention of the diseases they cause. A number of common features appear in this group of viruses:
SARS-CoV, MERS-CoV, and SARS-CoV-2 belong to β-coronaviruses.
They are zoonotic viruses that can cause infections in both humans and animals (23).
The natural reservoir host of the viruses are bats.
Before SARS-CoV, MERS-CoV, and SARS-CoV-2 become pathogenic to humans, a so-called species breakthrough has to occur twice: first, between bats and other mammals (vectors), then between the mammals and a human (22, 23).
The transmission of the disease occurs through direct contact with animals.
The intermediate hosts in case of SARS were palm civets, in case of MERS- camels and case of SARS-CoV-2- bats, and snakes (16, 22).
Indirect contact with excretions and secretions of animals is a source of infection as well, e.g., (milk) or consumption of improperly heat-treated meat.
The subinfections are transmitted by droplets and direct contact between humans.
The common feature of the discussed CoVs is the ability to cause acute viral pneumonia with the result of acute respiratory distress syndrome (ARDS), sepsis, septic shock, and patient death.
Coronaviruses are highly resistant to environmental conditions and can survive in aerosol form. This feature allows for unlimited dissemination in the environment and a high level of infectiousness. The infections can spread through the air-conditioning systems.
The transmission of infection through indirect contact (contaminated objects) through fecal-oral contact is also essential (29).
Currently, preventive measures are focused on isolating cases with confirmed infections in the form of quarantine, detecting infections in contact persons, and monitoring their condition.
10. Conclusions
At present, the efforts of scientists around the world are concentrated on the search for effective drugs and vaccines against COVID-19, but the expected preparation has not yet been found. Therefore, constant cooperation in this direction is essential.