1. Background
The potential of protozoan parasites for entry and replacement in the human lung has been reported in some studies (1, 2). Up to now, few cases of respiratory infections caused by parasites have been reported, which may be attributed to the difficulty associated with detecting parasitic agents in respiratory specimens. However, over recent years, there has been an increase in the reports of lung infections caused by parasites (3-5). Most parasitic lung infections are asymptomatic, but it has been reported that if the immune system is weakened, these infections can be accompanied by symptoms (1, 2). The most prevalent parasites causing lung infections are Toxoplasma, Cryptosporidium, and sometimes Acanthamoeba and Microsporidia (1, 2). Researchers have studied the effectiveness of several diagnostic methods for identifying these parasites. For instance, Niyyati et al. (6), Perez-Santonja et al. (7), and Boggild et al. (8) evaluated and compared different diagnostic methods for identifying Acanthamoeba keratitis. Saigal et al. (9) and Khanaliha et al. (10) assessed the direct microscopic and molecular methods for the identification of Microsporidia in fecal samples, and Ozkoc et al. (3) compared the same methods for the identification of the same parasite in bronchoalveolar lavage (BAL) samples.
Mercado et al. (11) compared the potential of the Ziehl Neelsen and molecular methods in the detection of Cryptosporidium in sputum samples. Bourdin et al. (12) investigated the potential of molecular and immunoblotting methods in the diagnosis of Toxoplasma in blood and ocular specimens of patients with ocular toxoplasmosis and compared them with the control group. Lavrard et al. (13) compared the efficiency of three diagnostic methods to detect pulmonary toxoplasmosis in BAL samples. To identify infectious agents in the lung, the appropriate sample is generally the secretion of the lower respiratory tract obtained through the BAL technique (14, 15). Various methods have also been used to identify parasites in lung specimens (2, 3). However, the value of these methods in detecting these parasites is yet to be elucidated in BAL specimens.
2. Objectives
In our country, studies on respiratory parasitic infections are scarce and far between. The present study aimed to evaluate and compare two diagnostic methods to detect some pathogenic protozoa in BAL samples obtained from immunocompromised patients with chronic obstructive pulmonary disease (COPD).
3. Methods
3.1. Sampling
Our study population included those who had both impairments of the immune system and respiratory disorders, approved by specialists. We excluded people who had no respiratory symptoms and people whose impairment of the immune system was not approved.
3.2. Respiratory Secretion Preparation
Written informed consent was obtained from the selected patients, and they completed a questionnaire containing the demographic information and the history of background disease. The pulmonary secretions of each patient were then obtained by the BAL technique (16). Fresh samples were immediately transferred to the laboratory, and the testing process was initiated on them.
3.3. Experiments
3.3.1. Direct Microscopic Diagnosis
Two smears were provided from each sample and stained by Ziehl Neelsen and Giemsa methods for direct microscopic diagnosis. The Ziehl-Neelsen stained smears were used to diagnose Cryptosporidium (1) and Microsporidia (17). The Giemsa stained smears were used to diagnose Toxoplasma (18).
3.3.2. PCR
The remaining samples were used for the PCR assay to diagnose the three protozoan parasites. First, the DNA was extracted by the phenol-chloroform method (19). Afterward, the genus-specific primers of four kinds of protozoa were used for PCR amplification. Table 1 presents the sequences of specific primers to diagnose the parasites. A Master Mix kit (CinnaGen Co.) was used to optimize the PCR assays. A final volume of 25 µL was used for amplification in an Eppendorf thermocycler (Eppendorf AG, Germany). The programs were used according to the following table (Table 2). Finally, the PCR products were assessed by 1.5% agarose under ultraviolet light. Of note, the positive control of Cryptosporidium and Microsporidia was kindly awarded by Dr. Majid Pirestani (Parasitology and Entomology Department, Tarbiat Modares University, Tehran, Iran), and Toxoplasma was provided by Dr. Saeedeh Shojaee (Department of Parasitology, Tehran University of Medical Sciences, Tehran, Iran).
| Parasite | Initial Denaturing Temperature (Time) | Number of Amplification Cycles | Denaturing Temperature (Time) | Annealing Temperature (Time) | Extension Temperature (Time) | Final Extension Temperature (Time) |
|---|---|---|---|---|---|---|
| Toxoplasma | 94°C (5 min) | 35 | 94°C (30 s) | 58°C (30 s) | 72°C (30 s) | 72°C (10 min) |
| Cryptosporidium | 94°C (5 min) | 32 | 94°C (45 s) | 55°C (40 s) | 72°C (60 s) | 72°C (7 min) |
| Microsporidia | 94°C (10 min) | 35 | 94°C (30 s) | 62°C (30 s) | 72°C (30 s) | 72°C (10 min) |
3.3.3. Sensitivity and Specificity Assessment
The sensitivity and specificity of the PCR-based diagnostic method were separately calculated and compared with the standard gold method of each parasite.
4. Results
In this study, we examined 64 patients with immunodeficiency accompanied by COPD. Table 3 presents the results of the examination of BAL specimens with direct and PCR methods to identify Toxoplasma, Cryptosporidium, and Microsporidia parasites. Noteworthy, Microsporidia was not identified in the samples using these two diagnostic methods.
| Direct Microscopic Diagnosis | PCR | |||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| Toxoplasma | 3 | 61 | 2 | 62 |
| Cryptosporidium | 9 | 55 | 2 | 62 |
| Microsporidia | 0 | 64 | 0 | 64 |
The sensitivity and specificity of the PCR-based method compared to the gold standard method for Toxoplasma and Cryptosporidium are summarized in Table 4. As observed, the sensitivity and specificity of the PCR-based method were 66.7% and 100% for Toxoplasma and 11.1% and 98.2% for Cryptosporidium.
| Direct Method (Gold Standard) | |||
|---|---|---|---|
| Positive | Negative | Total | |
| PCR (Toxoplasma) | |||
| Positive | 2 | 0 | 2 |
| Negative | 1 | 61 | 62 |
| Total | 3 | 61 | 64 |
| PCR (Cryptosporidium) | |||
| Positive | 1 | 1 | 2 |
| Negative | 8 | 54 | 62 |
| Total | 9 | 55 | 64 |
5. Discussion
The increase in the identification of various immune system disorders worldwide has revealed the incidence of more disseminated parasitic infections, such as pulmonary infections (24-26). These infections require special attention because significantly more immunocompromised cases have been reported in recent years (1). Misdiagnosis of these infections can lead to treatment failure and death (1). In this regard, the standardization of differential diagnostic methods seems to be the first step for identifying the types of parasites that cause respiratory infections. Today, there are facilities for direct microscopic examination (staining), and there exist culture and PCR-based methods for identifying different types of parasites (27). These methods are routinely used for the laboratory detection of COPD causative agents in the samples of pulmonary secretions (28). The efficiency of a diagnostic method may change based on the type of specimen; therefore, it is necessary to design and conduct studies to evaluate the effectiveness of various techniques for different types of specimens, like the current study.
In the present study, all BAL samples were examined by two methods to compare the sensitivity and specificity of each method for identifying different kinds of protozoa. Our observations showed that the sensitivity of PCR for identifying Toxoplasma and Cryptosporidium was 66.7% and 11.1%, respectively (Figure 1). Furthermore, Microsporidia was not detected in BAL samples by PCR and direct microscopic methods. In a study, Lavrard et al. (13) analyzed BAL samples from HIV patients for Toxoplasma infection. The researcher did not report any difference in the efficiency of direct microscopic and PCR methods in identifying this parasite, which is contrary to the present study, where the direct method was more sensitive.
Due to the similarity of the specimens and the diagnostic method in both studies, it seems that a human error led to the difference in the results. In another study, Morgan et al. assessed the potential of PCR and direct microscopic method (acid-fast staining) to identify Cryptosporidium in fecal samples. In contrast to our study, they showed that compared with routine microscopic methods, the PCR method was more efficient in identifying Cryptosporidium in this type of specimen (29). However, in a review study of respiratory cryptosporidiosis, Sponseller et al. (30) reached a conclusion similar to ours. More specifically, they reported that the direct microscopic methods (acid-fast and Giemsa) were more efficient than PCR in identifying Cryptosporidium in BAL samples. It seems that the difference between the samples (stool and BAL) led to the difference between the results of the present study and Morgan et al.’s study (29).
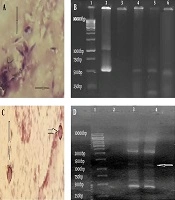
Identification of Toxoplasma and Cryptosporidium. A, Toxoplasma tachyzoites in the BAL sample by staining method (Giemsa staining, 100×); B, Toxoplasma identification by PCR method [Lane 1, marker (thermo-scientific SM0313); Lane 2, positive control; Lane 3, negative control; Lanes 4-6, positive samples with specific fragment for Toxoplasma (~529 bp)]; C, Cryptosporidium oocysts in the BAL sample by staining method (Ziehl Neelsen staining, x100); D: Cryptosporidium identification by PCR method [Lane 1, marker (thermo-scientific SM0313); Lane 2, negative control; Lane 3, positive control; Lane 4, positive sample with specific fragment for Cryptosporidium (~1325 bp)]
Recent studies have confirmed that PCR is an accurate method for identifying and determining Cryptosporidium species. Nonetheless, when the number of parasites is low, they cannot be detected by PCR. Primers also play a role in the sensitivity and specificity of the test. The newly designed primers seem to be effective in detecting a small number of Cryptosporidium parasites in fecal specimens (31). However, for more accurate conclusions, these primers should be tested on other samples, as well.
Saigal et al. (9) and Mena et al. (32) evaluated the direct microscopic and nested PCR methods in the detection of intestinal microsporidiosis. In their study, the sensitivity and specificity of the molecular method were reported to be higher than those of the staining methods. In both studies, two staining methods were compared to detect Microsporidia. Based on the results, the modified trichrome staining had more sensitivity and specificity compared to the calcofluor staining method. Ozkoc et al. (3) and Tabatabaie et al. (33) conducted a similar study on BAL samples and obtained similar results to the previous study. In our study, Microsporidia was not detected in any specimen. In Iran, the prevalence of Microsporidia in immunocompromised patients was 8.18% (34). Given that the gastrointestinal and pulmonary cases of Microsporidia are most often reported in immunocompromised individuals, it is possible that the result of our study was false-negative. Differences in the type of specimens, incorrect choice of staining method, and factors such as DNA extraction and examiner experience (in identifying Microsporidia) can be effective in obtaining such results.
5.1. Conclusions
Determining a standard diagnostic method for parasites depends on factors, such as the type of specimen and the type of parasite. Based on the results of the present study, the direct microscopic method is the best diagnostic method for Toxoplasma and Cryptosporidium in BAL samples. There was only a slight difference between the results of direct microscopic examination and PCR in the diagnosis of Toxoplasma; therefore, to achieve more accurate results, it is suggested that such studies be repeated with larger sample sizes and different specimen types.
![Identification of <i>Toxoplasma</i> and <i>Cryptosporidium</i>. A, <i>Toxoplasma</i> tachyzoites in the BAL sample by staining method (Giemsa staining, 100×); B, <i>Toxoplasma</i> identification by PCR method [Lane 1, marker (thermo-scientific SM0313); Lane 2, positive control; Lane 3, negative control; Lanes 4-6, positive samples with specific fragment for <i>Toxoplasma</i> (~529 bp)]; C, <i>Cryptosporidium</i> oocysts in the BAL sample by staining method (Ziehl Neelsen staining, x100); D: <i>Cryptosporidium</i> identification by PCR method [Lane 1, marker (thermo-scientific SM0313); Lane 2, negative control; Lane 3, positive control; Lane 4, positive sample with specific fragment for <i>Cryptosporidium</i> (~1325 bp)] Identification of <i>Toxoplasma</i> and <i>Cryptosporidium</i>. A, <i>Toxoplasma</i> tachyzoites in the BAL sample by staining method (Giemsa staining, 100×); B, <i>Toxoplasma</i> identification by PCR method [Lane 1, marker (thermo-scientific SM0313); Lane 2, positive control; Lane 3, negative control; Lanes 4-6, positive samples with specific fragment for <i>Toxoplasma</i> (~529 bp)]; C, <i>Cryptosporidium</i> oocysts in the BAL sample by staining method (Ziehl Neelsen staining, x100); D: <i>Cryptosporidium</i> identification by PCR method [Lane 1, marker (thermo-scientific SM0313); Lane 2, negative control; Lane 3, positive control; Lane 4, positive sample with specific fragment for <i>Cryptosporidium</i> (~1325 bp)]](https://services.brieflands.com/cdn/serve/3170b/2c8d70be269b6ae77155c56bf4ef2db4d9e0cc2a/jjm-111038-g001-F1-preview.webp)