1. Background
Streptococcus pneumoniae, known as pneumococci, can lead to various diseases ranging from sinusitis and acute otitis media to life-threatening diseases, including meningitis and bacteremia (1-3). Moreover, this bacterium circulates among human societies by colonizing the upper respiratory tract of healthy carriers, representing as its major reservoir (4). Infants and under five-year-old children are more susceptible to pneumococcal diseases due to the lack of a mature immune system and frequent colonization by pneumococci (5). Despite the availability of various vaccines and antibiotics, the World Health Organization (WHO) reported that invasive pneumococcal diseases were responsible for approximately 1.6 million deaths worldwide in 2008 (6).
The polysaccharide capsule, responsible for phagocytosis resistance in the absence of type-specific host antibodies, plays a major role in the invasion of S. pneumoniae in the systemic blood circulation (7). Based on the capsular composition, more than 90 serotypes of pneumococci have been recognized, among which a moderate number of serotypes are related to major infections (8). For the prevention of pneumococcal diseases, serotype-specific pneumococcal capsular polysaccharide vaccines and pneumococcal conjugate vaccines have been designed. These vaccines are composed of the capsular polysaccharides of the most prevalent virulent serotypes. Two types of vaccines are available in the market, including PCV-10 and PCV-13. The PCV-10 type covers various serotypes (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F) while immunity to 13 serotypes is provided by PCV-13 (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F(9).
The WHO recommends general immunization against this pathogen as the best preventive strategy against pneumococcal diseases (10). In Iran, a national vaccination program is not currently initiated, and people who want to be vaccinated for pneumococci must do it at their cost. Thus, we need widespread monitoring of serotype diversity of S. pneumoniae in Iran to determine the requirement of these vaccines. In recent decades, the treatment of pneumococcal diseases has been complicated due to the increasing penicillin-resistant S. pneumoniae strains (11). The first line of treatment for antibiotic therapy of pneumococcal infections is beta-lactam antibiotics. The first case of penicillin non-susceptible pneumococci (PNSP) was reported in 1967, and since then, resistant strains have been continuously increasing worldwide (12-14). Furthermore, the emergence of multi-drug resistant (MDR) S. pneumoniae isolates has made it more difficult to treat pneumococcal infections. The S. pneumoniae isolates resistant to three or more antimicrobial agents are defined as MDR (1, 15).
2. Objectives
Long-term local monitoring of antibiotic resistance in S. pneumoniae can be beneficial for the prevention and control of pneumococcal infections with the correct choice of antibiotics. Therefore, this study focused on the four-year surveillance of serotypes, antimicrobial resistance, and molecular epidemiology of S. pneumoniae strains isolated from blood cultures of children under five-years-old in Bojnurd, Northeastern Iran, between 2014 and 2018.
3. Methods
3.1. Sample Collection
This study was conducted from 2014 to 2018 in a major teaching referral Hospital of Bojnurd. All S. pneumoniae strains isolated from blood cultures of non-vaccinated under five-year-old children with non-focal fever suspected endocarditis and acute ill-health were included in this study. A total of 51 isolates were collected. Previously vaccinated children were not entered into this study. It should be noted that in this project, we did not perform additional sampling on patients and used laboratory samples after analysis.
3.2. Strain Identification
All blood samples were inoculated into blood culture vials and transported to the Medical Laboratory of Imam Reza Hospital. After 48-h incubation at 37°C, the positive cultures were subsequently inoculated onto sheep blood agar plates. Plates were incubated at 37°C in 5% - 10% CO2 overnight. Suspected colonies were identified based on alpha hemolysis, morphology, gram staining, optochin susceptibility (Oxoid, Basingstoke, UK), and bile solubility tests.
3.3. Antimicrobial Susceptibility Test
Susceptibility testing was performed against tetracycline (TET), oxacillin (OXA), trimethoprim-sulfamethoxazole (SXT), clindamycin (CLI), erythromycin (ERY), chloramphenicol (CHL), and vancomycin (VAN) for all isolates using the Kirby-Bauer disk diffusion method as per recommendations of the 2017 Clinical and Laboratory Standards Institute (CLSI) (16) using ROSCO antibiotic disks. The strains with an inhibition zone above 20 mm around the 1-µg oxacillin disk test were evaluated as penicillin-resistant. The MIC was determined for these isolates using cefotaxime E-test strips (Biomeriux, France). The interpretive breakpoint used to define penicillin non-susceptible (PNSP) isolates was ≥ 8 (penicillin parenteral, non-meningitis) using the E-test method (Biomeriux, France) (16). Besides, S. pneumoniae ATCC 49619 was used as the control strain. A MIC ≥ 8 µg/mL was considered resistant (11, 17, 18).
3.4. DNA Extraction
The total DNA of S. pneumoniae isolates was extracted using a Genet bio blood DNA extraction kit (Genet Bio, Korea). According to kit recommendations, lysozyme (Sigma, Germany) was added at a concentration of 20 IU/mL to the lysis buffer.
3.5. Polymerase Chain Reaction
We confirmed S. pneumoniae isolates using the polymerase chain reaction (PCR). The lytA and psaA genes were selected as marker genes to detect S. pneumoniae (19). The PCR was performed using a TAKARA gradient PCR TP600 thermal cycler (TAKARA, Japan). We used Emerald 2X premix Master Mix (TAKARA, Japan) for PCR. The PCR was performed using the following program: 5 min at 94°C for primary denaturation, followed by 35 cycles of amplification (denaturation at 94°C for 30 s; annealing at 58°C for 30 s; extension at 72°C for 30 s), and a final extension at 72°C for 7 min. The PCR products were evaluated using 1.5% agarose gel electrophoresis. Confirmed isolates were stored at -80°C in 15% sterilized glycerol containing Brain Heart Infusion Broth Media for further analyses (20).
3.6. Serotyping
Serotyping was performed using a Quelling reaction with Pneumotest-Latex according to the manufacturer’s instructions (Statens Serum Institute, Copenhagen, Denmark). For serotypes 6, 15, 19, and 23, we also performed additional PCR for specific serotypes (21). We set four multiplex PCRs for these serotypes based on the modified Marimon method (22). The PCR thermal cycling condition was 15 min at 95°C, followed by 35 cycles of 60 s at 95°C, 60 s at 57°C, and 60 s at 72°C, and a final extension step of 10 min at 72°C. The PCR products were evaluated using 1.5% agarose gel electrophoresis.
3.7. Multi Locus Sequence Typing
Multilocus sequence typing (MLST) was done using seven housekeeping gene targets, including aroE, gdh, gki, recP, spi, xpt, and ddl, as described elsewhere (1). The PCR products were subsequently sequenced. The PCR products were sequenced in both directions with an ABI 3730XL DNA analyzer. Sequences were submitted to the MLST database (http://pubmlst.org/spneumonia/), and the STs were determined.
3.8. Statistical Analysis
The statistical analysis was performed using Graph Pad Prism 8.0 (version 8.3.0 538). Differences or similarities were evaluated using a two-way ANOVA test. The P-values of ≤ 0.05 were considered statistically significant.
4. Results
4.1. Antimicrobial Susceptibility
Most of the S. pneumoniae isolates showed high resistance rates to erythromycin (88.2%), clindamycin (86.3%), trimethoprim-sulfamethoxazole (84.3%), and tetracycline (84.3%). The resistance rates to chloramphenicol (64.7%), Oxacillin (35.3%), and cefotaxime (11.8%) were relatively low. Notably, all isolates were susceptible to vancomycin. As shown in Table 1, the overall antibiotic resistance trend, except for clindamycin and vancomycin, was increasing from 2014 to 2018. The highest resistance in 2014 - 2015 was to tetracycline, in 2015 - 2016 to clindamycin, in 2016 - 2017 to cefotaxime and oxacillin, and in 2017 - 2018 to cefotaxime. However, the difference in resistance was not statistically significant between the years (P = 0.057).
| Antimicrobial Agents | Resistance, No. (%) | Total | |||
|---|---|---|---|---|---|
| 2014 - 2015 | 2015 - 2016 | 2016 - 2017 | 2017 - 2018 | ||
| VAN | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| TET | 12 (27.9) | 8 (18.6) | 10 (23.2) | 13 (30.2) | 43 (84.3) |
| CTX | 0 (0) | 0 (0) | 2 (33.33) | 4 (66.67) | 6 (11.8) |
| OXA | 1 (5.6) | 4 (22.2) | 6 (33.3) | 7 (38.9) | 18 (35.3) |
| ERY | 10 (22.2) | 11 (24.4) | 10 (22.2) | 14 (31.1) | 45 (88.2) |
| CLI | 8 (18.2) | 14 (31.8) | 9 (20.4) | 13 (29.5) | 44 (86.3) |
| SXT | 4 (9.3) | 11 (25.5) | 14 (32.6) | 14 (32.6) | 43 (84.3) |
| CHL | 3 (9.1) | 9 (27.3) | 9 (27.3) | 12 (36.3) | 33 (64.7) |
Antimicrobial Resistance Between 2014 and 2018
Antibiotic resistance levels were different in PNSP strains compared to PSSP strains. In the PSSP isolates, resistance against chloramphenicol was 51.6%, trimethoprim-sulfamethoxazole 90.8%, clindamycin 72.6%, erythromycin 93.1%, tetracycline 74.2%, and cefotaxime 6.9%, but in PNSP isolates, resistance to trimethoprim-sulfamethoxazole was 77.8%, clindamycin 100%, erythromycin 83.3%, tetracycline 94.4%, and cefotaxime 16.7%. The difference in resistance was not statistically significant between PNSP and PSSP isolates (P = 0.2227). Between different serotypes, the highest resistance to chloramphenicol was observed in 15A, 14, and 19A. All detected serotypes were highly resistant to sulfamethoxazole, clindamycin, erythromycin, and tetracycline. The 19F and 15A serotypes had low resistance to oxacillin, and all detected serotypes had low resistance to cefotaxime (Table 2). It should be noted that the relationship between serotype and antibiotic resistance was not statistically significant (P = 0.2865).
| 23F | 19A | 19F | 1 | 6A/B | 14 | 15A | 15B/C | NT | |
|---|---|---|---|---|---|---|---|---|---|
| CHL, % | 57.1 | 80 | 60 | 0 | 55.6 | 80 | 100 | 60 | 100 |
| SXT, % | 85.7 | 90 | 100 | 0 | 77.8 | 80 | 0 | 80 | 100 |
| CLI, % | 85.7 | 90 | 80 | 0 | 77.8 | 80 | 100 | 80 | 100 |
| ERY, % | 85.7 | 100 | 60 | 87.5 | 88.9 | 100 | 100 | 80 | 100 |
| OXA, % | 42.9 | 30 | 100 | 12.5 | 11.1 | 40 | 100 | 20 | 100 |
| CTX, % | 14.3 | 20 | 20 | 25 | 0 | 0 | 0 | 0 | 0 |
| TET, % | 71.4 | 90 | 80 | 100 | 77.8 | 80 | 100 | 80 | 100 |
Antimicrobial Resistance of 51 Streptococcus pneumoniae Blood Culture Isolates Against Eight Common Antimicrobial Agents Among Different Serotypesa
4.2. Serotype
Among a total of 51 S. pneumoniae isolates, 50 (98%) isolates were typable. The most common serotypes were 19A (19.6%), 6A/B (17.64%), 1 (15.68%), 23F (13.72%), 19F, 14 and 15B/C (9.8%), and 15A (1.96%) but in PNSP isolates, the most common serotypes were 19F, 19A, 23F, 14, and finally 15A, 6A/B, 1 and 15B/C, in sequence. Some serotypes such as 19F and 15A were just found in PNSP isolates, and some resistances were probably serotype-dependent. The coverage rates of PCV10 and PCV13 were 66.64% and 86.24%, respectively.
4.3. Multi Locus Sequence Typing
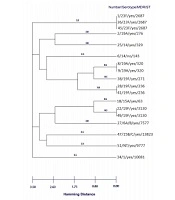
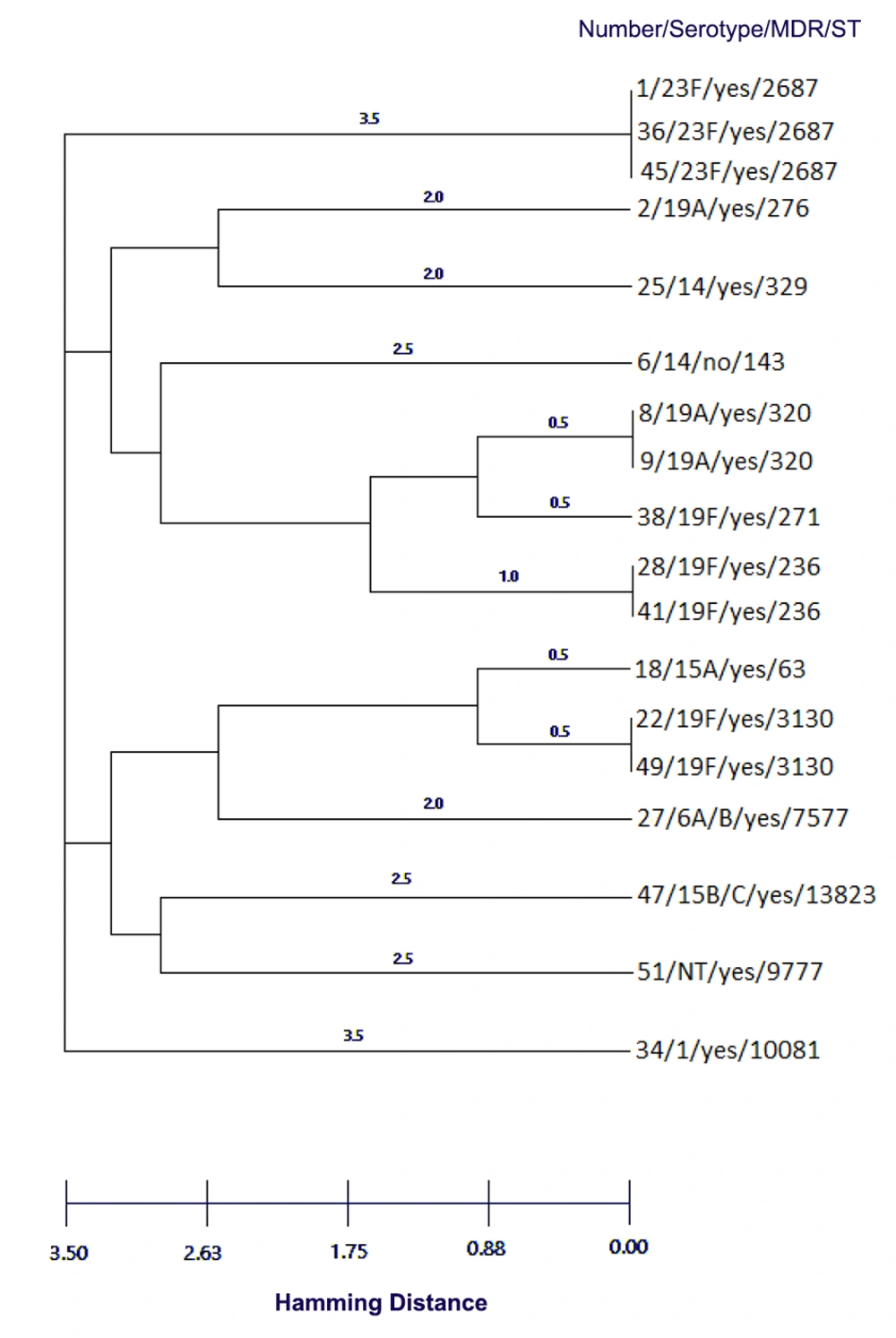
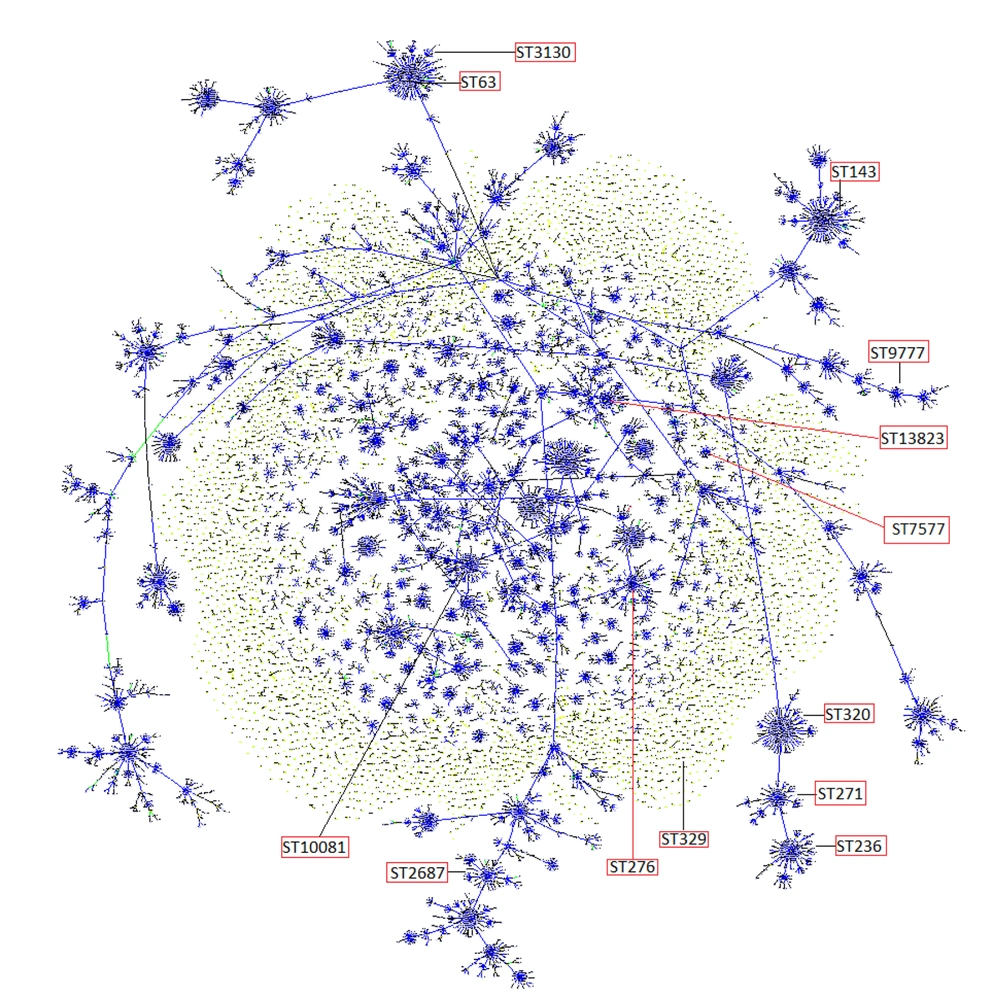
Due to the importance of penicillin non-susceptible pneumococci (PNSP) isolates, we performed Multi Locus Sequence Typing on all of these 18 isolates [f]. The three isolates belonged to international clones Sweden15A-25-19A, Taiwan19F-14-1, and Taiwan19F-14. Two isolates were double locus variant (DLV) of Taiwan19F-14 (ST 320), and one isolate was single locus variant SLV (ST271) of this clone. These isolates belonged to serotypes 19A, 1, 14, and 6A/B. The four predominant sequence types (ST2687, ST320, ST236, and ST3130) accounted for 50% of the PNSP isolates (Table 3). Our major complex was clonal complex 0 comprising three STs (236, 271, and 320) with five isolates and clonal complex 1 comprising two STs (63 and 3130) with three isolates. The complete eBURST analysis results are presented in Figures 1 and 2. The differences in antibiotic resistance between various STs were not statistically significant (P = 0.0636). The distribution of STs was not statistically significant in different serotypes (P = 0.9990).
| Numbers | Serotype | Antimicrobial susceptibilitya | MDR | MIC, µg/mLb | Sequence Type | Clonal Complexc | International clone | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TET | CTX | OXA | ERY | CLI | SXT | CHL | |||||||
| 1 | 23F | R | S | R | R | R | R | R | yes | 8 | 2687 | 7 | - |
| 2 | 19A | R | S | R | R | R | R | R | yes | 8 | 276 | 3 | - |
| 6 | 14 | R | S | R | R | R | S | R | no | 16 | 143 | 0 | - |
| 8 | 19A | R | R | R | R | R | R | R | yes | 8 | 320 | 0 | DLV of Taiwan19F-14 |
| 9 | 19A | R | R | R | R | R | R | R | yes | 16 | 320 | 0 | DLV of Taiwan19F-14 |
| 18 | 15A | R | S | R | R | R | S | R | yes | 8 | 63 | 0 | Sweden15A-25-19A |
| 22 | 19F | R | S | R | S | R | R | S | yes | 8 | 3130 | 0 | - |
| 25 | 14 | R | S | R | R | R | R | R | yes | ≥ 32 | 329 | 832 | - |
| 27 | 6A/B | R | S | R | R | R | S | S | yes | ≥ 32 | 7577 | 2029 | - |
| 28 | 19F | R | S | R | R | R | R | R | yes | 16 | 236 | 0 | Taiwan19F-14-1 |
| 34 | 1 | R | S | R | R | R | R | R | yes | 8 | 10081 | 84 | - |
| 36 | 23F | R | S | R | R | R | R | R | yes | 16 | 2687 | 0 | - |
| 38 | 19F | S | R | R | R | R | R | R | yes | 8 | 271 | 0 | SLV of Taiwan19F-14 |
| 41 | 19F | R | S | R | R | R | R | R | yes | ≥ 32 | 236 | 0 | Taiwan19F-14 |
| 45 | 23F | R | S | R | R | R | R | R | yes | 16 | 2687 | 0 | - |
| 47 | 15B/C | R | S | R | S | R | S | S | yes | 16 | 13823 | 100 | - |
| 49 | 19F | R | S | R | S | R | R | S | yes | ≥ 32 | 3130 | 0 | - |
| 51 | NT | R | S | R | R | R | R | R | yes | 8 | 9777 | 0 | - |
Sequence Types of Penicillin Non-susceptible Streptococcus pneumoniae Isolates
Dendrogram constructed from MLST data showing the genetic relatedness of the 18 pneumococcal sequence types in Table 3. ST320 and ST276 are SLVs of ST271, and also ST63 and ST3130 are SLV.
Population snapshot of 12 STs of Streptococcus pneumoniae isolates. Clusters of related STs and individual unlinked STs with the entire S. pneumoniae MLST database (15219 STs) are displayed as a single eBURST diagram by setting the group definition to 0 of 7 shared alleles. STs found in this study are labeled and other ST labels have been removed. Our STs belonged to CC0, CC3, CC84, CC100, CC832, and CC2029.
5. Discussion
Streptococcus pneumoniae is one of the leading causes of pediatric and adult infections. The serotype distribution, antimicrobial resistance, and genotypes vary across different populations and change over time in each geographical region. Although the results of such studies seem to be of local use, they not only can improve global health by affecting pneumococcal vaccine immunization programs but also raise global awareness about antimicrobial resistance and molecular aspects of pneumococcal infections. This four-year surveillance study revealed that the most common serotypes of blood-isolated pneumococci in our region were 19A, 1, 23F, 19F, 14, and serogroups 15 (B/C) and 6 (A/B), which look like other countries before pneumococcal conjugate vaccination (15, 23, 24). The PCV10 and PCV13 types covered 66.64% and 86.24% of serotypes, respectively, which, like other studies, illustrates that PCV13 could be suitable for controlling S. pneumoniae isolates in this region (25).
All of our isolates were highly resistant to tetracycline, erythromycin, clindamycin, trimethoprim-sulfamethoxazole, and chloramphenicol. Contrary, most isolates were susceptible to cefotaxime and penicillin. Of importance, all isolates were susceptible to vancomycin. The highest antimicrobial resistance rate was seen for the 1 and 19A serotypes, while the 6A/B serotype showed the lowest resistance. Table 1 shows that, except for vancomycin, the antimicrobial resistance rate followed an increasing pattern from 2014 to 2018. Penicillin-resistant S. pneumoniae emerged as a global problem in the past decade, and it seems that beta-lactam resistance was increasing during the last few years. In the present study, 35.3% of the isolates were PNSP, while the overall prevalence of PNSP was lower in other studies. Its prevalence in Morocco was 22.2% from 2007 to 2014 (26). In other studies, the resistance rate to penicillin in S. pneumoniae was 28% and 20% in Tehran, Iran, and in non-meningitis isolates of pneumococci was 10.7% in Shanghai, China (1, 2, 25).
Some studies proved that PCV vaccination led to a decrease in the prevalence of antibiotic-resistant strains. Diawara et al. (26) showed that the resistance rate to penicillin decreased from 34.5% to 22.9% after PCV vaccination in children. The reduction in the PNSP rate after PCV vaccination was reported in several countries (23, 27-29). Regarding relatively high resistance against penicillin in our isolates and also high serotype coverage of the 13-valent vaccine, it seems that the PCV vaccination could be useful to prevent the spread of resistant strains. The most prevalent serotypes among our PNSP were 19F, 19A, 23F, 14, 15B/C, 6A/B, and 1. No meaningful correlation was observed between serotypes and penicillin resistance (P > 0.05). These serotypes were mainly covered by PCV-13.
Serotypes 14, 6A/B, and 19F were observed among highly resistant isolates with penicillin MICs ≥ 32. Dissemination of 19F isolates has increased in many parts of the world, and they have become resistant to penicillin (30-32). In this study, all 19F isolates were resistant to penicillin. It should be noted that regardless of antimicrobial resistance, the most prevalent serotypes among our isolates were 19A, 6A/B, 1, 23F, 19F, 14, 15B/C, and 15A, respectively. In a similar study in Trinidad and Tobago, the most prevalent serotypes among invasive isolates were 19F, 6B, 23F, 3, 19A, 6A, 14, and 9V, respectively (33). In another study in China, the most common serotypes were 19F, 19A, 15, 6B, 6A, and 17 (34). Comparing our results with other studies shows partial differences in various geographical areas (3, 24, 35-39); for example, serotype 1 was not reported in China and Trinidad and Tobago studies, but we found it in our isolates. Conversely, serotypes 3 and 17 were found in that two countries though we did not observe it in our study.
The MLST analysis of S. pneumoniae isolates in different countries showed that some international clones such as Spain23F-1, Spain6B-2, or England14-9 are circulating worldwide (1, 40, 41). The MLST analysis of PNSP isolates in this study revealed that three highly penicillin-resistant isolates with MIC ≥ 16 belonged to international clones, Sweden15A-25-19A (ST63), Taiwan19F-14-1, and Taiwan19F-14 (ST236). Importantly, for our Taiwan19F-14-1 and Taiwan19F-14 isolates, the MICs were higher than previously reported (41). In primary introduced Taiwan19F-14-1 and Taiwan19F-14, the penicillin MIC was 2, but in our isolates and variants, the MIC was minimally ≥ 8. Other prevalent STs in our isolates were ST2687, ST320, and ST3130. It should be noted that ST320 is a DLV, and ST271 is an SLV of the ST236 Taiwan19F-14 clone (Figure 1). The spread of ST320 can be problematic because it is an international virulent MDR strain (42). This strain has been reported in many countries and is commonly found in North America, Europe, China, and other Asian countries (2, 15, 42, 43). All of our ST320 isolates belonged to serotype 19A (Figure 1).
In the present study, 82.35% of the isolates were classified as MDR. The relatively high presence of Taiwan19F-14 clones and their related STs put them as significant clones in circulation. Talebi et al. (2) reported that common STs in erythromycin-resistant isolates of pneumococci were ST3130, ST180, and ST81. According to the results of another study by Raddoui et al. (44), ST81 was the most prevalent sequence type in macrolide-resistant S. pneumoniae isolates. Between 2007 and 2013, the most common ST in MDR pneumococci isolates was ST320 in Canada (15). Lucas et al. (33) reported that ST138, ST36, and ST180 were the most common sequence types in Trinidad and Tobago, while in this study, the most common STs in MDR isolates were 2687, 320, 236, 3130, 271, 329, 63, 7577, 10081, 13823, and 9777, respectively.
Serotype switching was observed in serotype 19A PNSP belonging to ST320 and ST276, serotype 19F distributed in ST3130, ST271, and ST236, and serotype 14 found in ST329 and ST143. A high rate of capsular switching was reported in other studies, especially in regions with national vaccination programs. Interestingly, we observed a high level of serotype switching in our isolates, although, in Iran, the state-run vaccination program has not been carried out yet. Therefore, further studies are required to confirm the relationship between serotype switching and vaccination. Finally, it seems that our PNSP isolates belonged to diverse clones that may be due to the various races living in this geographical area (Figure 2).
5.1. Conclusions
In conclusion, the current study showed that serotypes 19A, 6A/B, 1, 23F, 19F, 14, and 15B/C were commonly isolated from blood cultures of children under five-years-old. It seems that PCV-13 with the 86.24% coverage of blood isolates of pneumococci is the most suitable choice for vaccination in this region. Other important points are a relatively high prevalence of PNSP and MDR strains between 2014 and 2018. The MLST surveillance showed that the Taiwan19F-14 clone and its related STs played an essential role in disseminating resistant S. pneumoniae isolates in Bojnurd, Iran. Hence, to select the optimal antimicrobial treatment, to understand the distribution pattern of clones, and to have the most effective immunization program for S. pneumoniae-related diseases, long-term regional surveillance could be beneficial.