1. Background
Ventilator-associated pneumonia (VAP), a subset of hospital infections, occurs in patients who have been mechanically ventilated for at least 48 hours (1, 2). Ventilator-associated pneumonia is considered a common, serious, costly complication in hospitalized patients, which ranks first in hospital infections at intensive care units, with a high mortality rate (3). Ventilator-associated pneumonia that occurs between 48 and 72 hours after tracheal intubation is introduced as early-onset pneumonia; this type of pneumonia is often a result of aspiration, which complicates the process of intubation and is often formed due to the sensitivity of bacteria to antibiotics (e.g., methicillin-sensitive Staphylococcus aureus (MSSA), Homophiles influenza, and Streptococcus pneumoniae) (4). Ventilator-associated pneumonia that occurs after five days of mechanical ventilation is known as late-onset pneumonia and is often formed by antibiotic-resistant pathogens (such as methicillin-resistant S. aureus (MRSA), Pseudomonas aeruginosa, Acinetobacter species, and Enterobacter species) (4).
Risk factors related to hospital-acquired pneumonia include the age of over 45 years, heart ischemic disease, chronic obstructive pulmonary disease, kidney failure, dialysis, addiction to narcotic drugs, receiving antibiotics before the occurrence of pneumonia, smoking, previous hospitalization, coma, diabetes, blow to head, nasogastric tube, corticosteroid consumption, tracheostomy, mental status disorder, and mechanical ventilation (4, 5).
To diagnose the etiology, a positive culture of lower respiratory tract secretions is needed. The most important sampling techniques are endotracheal tube aspiration (ETTA), bronchoalveolar lavage (BAL), and protected specimen brushing (PSB) (4, 6). Endotracheal tube aspiration has a very high sensitivity (90 - 100%) so that it is considered a gold standard. The length of the treatment period is also important. According to studies conducted, most patients with VAP who have received suitable empirical antibiotic therapy had a good clinical response within the first six days of treatment (7). Long-term treatment can lead to colonization with resistance to antibiotics. Patients who receive a shorter period of antibiotic treatment show better clinical outcomes than those with longer treatment periods (8).
2. Objectives
As multi-drug resistant (MDR) strains are increasing day by day and there is a high mortality rate of VAP, the awareness of the occurrence of VAP, identification of causing pathogens, and their antimicrobial susceptibility patterns are necessary to improve and promote the hospital infection control system. This, in turn, will help employ effective methods of prevention, control, and improvement of empirical treatments, as well as reducing VAP incidence and resultant mortality. Our study aimed at determining the frequency and antibiotic susceptibility patterns of bacteria causing VAP in teaching hospitals of Shiraz.
3. Methods
3.1. Selection of Patients
This was a descriptive cross-sectional study performed on all patients who had been hospitalized in the internal wards and ICUs of teaching hospitals of Shiraz and had acquired VAP during their hospitalization within eight months (November 2017 to June 2018). This study aimed to determine the frequency of bacteria causing VAP and their antimicrobial susceptibility patterns. All patients who had no symptoms of pneumonia at the time of intubation but had pneumonia at least 48 hours after intubation were included in the study. The diagnostic criteria for having VAP were considered in the presence of at least two of the following cases: A fever above 38°C (hypothermia), leukocytosis or leukopenia, respiratory purulent secretions, a new or progressive infiltrate, consolidation or cavitation in chest radiography (3).
3.2. Identification of Isolated Bacteria
The patient’s endotracheal tube aspiration inside Falcon under sterile conditions was transferred to the laboratory of Shiraz School of Medicine. Then, the specimens were cultured and isolated on blood agar, MacConkey agar (in the aerobic atmosphere), and chocolate agar (with 5% - 10% CO2 pressure). All culture media were from HiMedia, India. At the same time, to check the possible contamination and identify the morphotype of organisms, Gram staining was done. After purification, the samples were Gram re-stained and the primary diagnostic tests (catalase and oxidase tests) were performed. To detect Gram-positive cocci, production of catalase, coagulase, DNase, and oxidase enzymes, Mannitol fermentation, and susceptibility to bacitracin, optochin and novobiocin, bile esculin test, (6.5% Nacl), Type of hemolysis (α, β, and γ) was checked. To detect of MRSA, 30 µg cefoxitin disk was also used.
The API 20 E kit (bioMerieux, UK) was used to detect Gram-negative bacilli. However, to detect non-fermentative Gram-negative bacteria such as Acinetobacter and Pseudomonas species, differential culture media were separately prepared and tested for samples. In addition, the TSI medium was used for all Gram-negative bacteria to examine the lactose and glucose fermentation patterns, as well as gas and H2S production, and the SIM medium was used to examine the motility of bacteria. For the final detection of the oxidase-negative and sugar- fermentative Gram-negative bacilli (Enterobacteriaceae), the website of APIWEB (WWW.biomerieux-usa.com/clinical/api) was used. For non-fermentative Gram-negative bacteria such as Pseudomonas and Acinetobacter, the website of ABS (www.tgw1916.net) was used. To verify the species of Acinetobacter baumannii, a PCR confirmation test on the bla OXA-21 gene was used, with primers F: 5’-TAATGCTTTGATCGGCCTTG-3’ and R: 5’-TGGATTGCACTTCATCTTGG-3’ (9). The primers were prepared from Novin Gene Company, Iran.
3.3. Antibiotic Susceptibility Assay
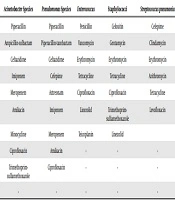
The disk diffusion (Kirby-Bauer) method was used to test the sensitivity to different antibiotics. Antibiotics that were used for antibiograms are listed in Table 1. Antibiotics were from HiMedia, India. The results of the inhibition zone were interpreted according to the CLSI 2018 standards, and SPSS16 software was used for statistical analysis of the data.
| Enterobacteriaceae | Acinetobacter Species | Pseudomonas Species | Enterococcus | Staphylococci | Streptococcus pneumonia |
|---|---|---|---|---|---|
| Amikacin | Piperacillin | Piperacillin | Penicillin | Cefoxitin | Cefepime |
| Ciprofloxacin | Ampicillin-sulbactam | Piperacillin-tazobactam | Vancomycin | Gentamycin | Clindamycin |
| Piperacillin | Ceftazidime | Ceftazidime | Erythromycin | Erythromycin | Erythromycin |
| Piperacillin tazobactam | Imipenem | Cefepime | Tetracycline | Tetracycline | Azithromycin |
| Imipenem | Meropenem | Aztreonam | Ciprofloxacin | Ciprofloxacin | Tetracycline |
| Meropenem | Amikacin | Imipenem | Linezolid | Trimethoprim-sulfamethoxazole | Levofloxacin |
| Fluoxetine | Minocycline | Meropenem | Teicoplanin | Linezolid | |
| Ceftazidime | Ciprofloxacin | Amikacin | - | - | - |
| Cefepime | Trimethoprim-sulfamethoxazole | Ciprofloxacin | - | - | - |
| Ceftriaxone | - | - | - | - | - |
4. Results
4.1. Basic Information
A total of 51 patients with VAP (hospitalized in the internal wards and internal ICUs of teaching hospitals of Shiraz) were sampled. After culture, 47 bacteria and two yeasts grew in media, and two samples did not grow. Therefore, 47 patients were included in the study. Yeast strains were excluded from the next process. The average age of the patients was 66 (range, 25 - 98 years); the average hospitalization period was 13.44 days (range, 3 - 45 days), and the average mechanical ventilation period was 11.73 days (range, 2 - 45 days). In this study, the mortality rate was 38%. Different reasons for hospitalization included: 10 cases of chronic obstructive pulmonary disease, eight cases of acute respiratory distress syndrome, seven cases of urosepsis, five cases of brain stroke, five cases of heart disease, two cases of sepsis, two cases of surgery, one case of cesarean section, one case of asthma, one case of lung fluid accumulation , one case of diabetes, one case of lung cancer, one case of blood cancer, one case of N1H1 influenza, and one case of kidney failure.
In this study, the late-onset VAP (61.7%) was more prevalent than the early-onset VAP (38.3%). The average calculated Clinical Pulmonary Infection score (CPIS) of patients was 8 (range, 6 - 10). The most commonly used empirical antibiotics were meropenem (76.6%) and vancomycin (78.7%). These two antibiotics were used in most cases as combined antibiotics. Information on the primary antibiotic treatment is presented in Table 2. As shown, 47 patients were included in the present study (two patients were excluded from the study due to the lack of culture growth and two patients due to yeast growth in clinical samples).
| Variable | All | Death, No. (%) | P-Value |
|---|---|---|---|
| Empirical antibiotic treatment | - | ||
| Single antibiotic | 8 | 4 (50) | |
| Combined antibiotics | 39 | 14 (35) | |
| Appropriateness of antibiotic treatment | 0.043 | ||
| Appropriate | 27 | 7 (25) | |
| Inappropriate | 20 | 11 (55) | |
| Group of initial antibiotic treatment | - | ||
| Early-onset VAP | 18 | 5 (27) | |
| Late-onset VAP | 29 | 13 (44) |
4.2. Organisms Causing VAP and Antimicrobial Susceptibility Tests
Overall, out of 59 bacteria that were isolated from 51 patients, 44 were Gram-negative and 15 were Gram-positive. Besides, 14 (30%) were poly-microbial and 33 (70%) were single-microbial. The most prevalent organisms were A. baumannii and P. aeruginosa. In this study, four methicillin-resistant Staphylococcus aureus were also isolated. Of the isolated bacteria, 19 were related to the Acinetobacter species, and 17 belonged to A. baumannii, as confirmed by the molecular PCR method using bla oxa-51 like replication gene. The results of the antimicrobial susceptibility of the Enterobacteriaceae family (14 bacteria) showed that the highest sensitivity of the Enterobacteriaceae family was to meropenem (11 bacteria), imipenem (eight bacteria), and ciprofloxacin (eight bacteria). The highest antimicrobial susceptibility of Pseudomonas species (11 bacteria) was to meropenem (eight bacteria), imipenem (seven bacteria), amikacin (six bacteria), and ciprofloxacin (six bacteria), and the highest resistance was to piperacillin-tazobactam (seven bacteria), ceftazidime (nine bacteria), and cefepime (six bacteria). The highest antimicrobial susceptibility of Acinetobacter species (19 bacteria) was to meropenem (11 bacteria) and amikacin (11 bacteria) and the highest resistance was to trimethoprim-sulfamethoxazole (13 bacteria), minocycline (13 bacteria), and ceftazidime (13 bacteria). The antimicrobial susceptibility test of Gram-positive bacteria showed that the highest sensitivity was to linezolid and the highest resistance was to erythromycin.
5. Discussion
Ventilator-associated pneumonia is one of the prevalent infections with high mortality in patients hospitalized in ICUs in most parts of the world. The prevalence of VAP in different regions is different according to several studies conducted (4, 10, 11). In the present study, the incidence of VAP was lower in teaching hospitals of Shiraz (especially Namazi Hospital). The reasons for the low incidence are as follows: (1) In the ICU of Namazi hospital, the ratio of the number of nurses to the number of patients is 1:1, while in a study in India (12), the ratio was 3:17; (2) Namazi Hospital has an active infection control committee that highly emphasizes the hygiene of the personnel, especially washing the hands; (3) care and protection of patients are done very carefully and patients are suctioned on time; (4) traveling in the ICU is prohibited, and patients are not accompanied by anyone else, except the nurse and the doctor.
Determining the factors that affect the extent of mortality will provide a better prognosis (13). In our study, 45.2% of the patients with VAP received antibiotics before the incidence of pneumonia, of whom 31.6% died while of the remaining 54.8%, only 8.7% died. In other words, patients who received antibiotics before the incidence of pneumonia had higher mortality. On the other hand, the most common microorganisms isolated from patients were Acinetobacter (28.8%) and Pseudomonas (18.6%), which are considered MDR pathogens. Probably, antimicrobial treatment before the onset of VAP has led to the selection of these MDR pathogens (14).
In Asian countries, the most common pathogenic agent of VAP in ICU patients is Acinetobacter, but in some Asian countries, such as Taiwan and Korea, it is commonly known as the second most common pathogen. In Korea and Taiwan, MRSA is considered the most common pathogenic agent of NP in patients hospitalized in the ICU so that in Korea, it accounts for 80-90% and in Taiwan, it includes 73% of all isolates of S. aureus (8). In general, the incidence of Acinetobacter is higher in Asian countries than in European countries while the incidence of MRSA and P. aeruginosa is lower in Asian countries than in European countries (15). The reason for the high incidence of Acinetobacter infection in Asian countries is not clear, but it may be due to temperature and humidity differences because the warmer the environment and the higher the moisture, the better the conditions for the growth of Acinetobacter (16).
In our study, the mortality rate was 23.4% for 20 individuals who received inappropriate empirical treatment and 14.9% for 27 participants who received appropriate empirical treatment (p value less than 0.05). Therefore, there was a significant relationship between the quality of treatment and mortality. Several clinical studies have shown that empirical treatment of VAP with an appropriate antimicrobial regimen is associated with lower mortality (17-19). In other words, the failure of antibiotic treatment is due to the presence of MDR pathogens (20). In a prospective study by Michel et al. (8) and a retrospective study by Green (21), the changes in antibiotics after providing the culture response did not diminish the extent of mortality in patients whose empirical antibiotic therapy was inappropriate. Therefore, if empirical antibiotic treatment is selected appropriately and timely, it can improve treatment outcomes (8). In our study, patients who had received inappropriate empirical treatment had a higher mortality rate; however, one should take into consideration that these patients had a critical condition of disease at the time of admission. Therefore, it cannot be properly demonstrated if inappropriate empirical treatment of hospital pneumonia increased their mortality or it was associated with their critical condition.
In our study, overall, 70.6% of isolated Acinetobacter species were sensitive to meropenem and 76.5% were sensitive to amikacin; in other words, they are the best coating for Acinetobacters. In the case of Pseudomonas, the highest susceptibilities were observed for meropenem, imipenem, and ciprofloxacin (90%, 80%, and 70%, respectively) and they appeared to be the best treatment against these bacteria. Meropenem seems to be the best coating for both bacteria. Also, in our study, the sensitivity of Gram-positive bacteria to linezolid was 100%. However, it is better to use vancomycin for treatment because linezolid is an alternative drug for treating VAP resulting from MRSA. If a patient has renal failure or receives drugs that cause renal toxicity, linezolid is preferable to vancomycin because it is difficult to determine and adjust the dose of vancomycin in patients with renal failure and it needs frequent monitoring of its blood levels (4). Therefore, it is suggested that vancomycin be used in the empirical treatment of MRSA, which is considered the standard treatment for MRSA, and linezolid be stored for specific cases. To make the study more precise, the samples needed to be taken before the administration of antibiotics, but usually, the patients immediately received broad-spectrum antibiotics. The low sample size was due to the lack of proper cooperation of nurses and doctors to collect samples.
5.1. Conclusions
Our data showed that most isolates (57%) were related to MDR pathogens. Probably, antimicrobial treatment before the onset of VAP led to the selection of these MDR pathogens. The most common organisms in the last study in Shiraz were A. baumannii, followed by MRSA and P. aeruginosa, but in our study, P. aeruginosa ranked second.