1. Background
Candidiasis is a fungal infection caused by Candida spp. that affects the mucosa and internal organs in humans (1). Candida albicans is an important yeast species, that colonizes the vaginal and oral mucosa of apparently healthy women. However, it can become pathogenic if and when the balance between yeast and mucous membranes and host defense mechanisms is disrupted (2-4). The World Health Organization and Food and Agriculture Organization define probiotics as microorganisms that exert beneficial effects on host health when present in adequate quantities, particularly by facilitating the maintenance of gastrointestinal health and digestion (5). Most probiotics are a part of the human mucosal microbiota, and are effective in preventing and treating atopy, eczema, dermatitis, and diarrhea as well as in treating inflammatory enteritis.
Probiotics also play an important role in maintaining the vaginal environment in healthy women. Meanwhile, the biofilm, in which microorganisms aggregate, exhibits self-protection, and the microorganisms communicate with each other via antibiotic resistance and exhibit social behavior through horizontal gene transfer and quorum sensing mechanisms. Probiotic strains secrete antagonists such as surfactants, bacteriocins, exopolysaccharides, organic acids, lactic acid, fatty acids, enzymes, and hydrogen peroxide, and reduce the biofilm biomass by changing the pH and initiating nutrient competition, which prevents biofilm formation by pathogens (6-10).
In a previous study, 140 probiotic strains were isolated from 35 types of Korean kimchi using 16S rRNA sequencing. Among them, Lactobacillus plantarum strains exhibiting antimicrobial activity and broad antibiotic resistance were selected (11). The growth of the microorganisms exhibiting highest pathogenicity, including C. albicans, was found to be almost completely inhibited in the mixed culture containing the probiotic strains and six pathogenic microorganisms (12). In the hydrogen peroxide production test (unpublished), 43 of 140 probiotic strains (30.7%) isolated from kimchi produced H2O2, among which 25 of 53 L. plantarum strains (47.2%) produced H2O2. This was attributed to direct inhibition via lactic acid, hydrogen peroxide, and bacteriocin production and low pH induced by probiotics (12). In a previous study, 140 probiotic strains were isolated from commercially available kimchi in Korea; these bacteria were phylogenetically identified based on their 16S rRNA gene sequences and examined for resistance to 18 antibiotics (11). Examination of the inhibitory effects of the probiotics in ME-180 cervical carcinoma cells revealed that the growth of C. albicans was inhibited in ME-180 cultures initially inoculated with L. plantarum at relatively high concentrations, regardless of the concentration of the C. albicans culture (12).
2. Objectives
In our previous study, probiotics exhibiting resistance to broad-spectrum antibiotics and high levels of inhibitory activity against pathogenic bacteria were isolated. In this study, the inhibitory effects of L. plantarum on C. albicans were examined in ME-180 cervical carcinoma cell cultures to determine the potential of L. plantarum as an auxiliary treatment agent for vaginitis.
3. Methods
3.1. Bacterial Strains and Cells
The C. albicans strain KCTC 7752 was obtained from the Korean Collection for Type Cultures. The L. plantarum strain isolated from commercially available kimchi (in Korea) was stored at 4 °C in the laboratory (11). Human cervical cancer ME-180 cells (KCLB30033) were used for the cell culture experiments.
3.2. ME-180 Cell Culture and Inoculation with Lactobacillus plantarum and Candida albicans
Cell culture was performed as described previously (13, 14). RPMI 1640 (Welgene, Daegu, Korea) supplemented with 10% fetal bovine serum (Welgene, Korea) was used as the culture medium. ME-180 cells (KCLB 30033, Korean Cell Line Bank, Korea) were inoculated with L. plantarum at 104, 106, and 108 colony-forming units per milliliter (CFU/mL). Following this, the ME-180 cells were seeded into a 24-well plate (SPL Life Sciences, Gyeonggi-do, Korea) at a density of 2 × 105 cells/mL, followed by overnight incubation at 37°C in a 5% CO2 incubator. The culture medium was replaced with fresh medium the following day, and C. albicans was used to inoculate the cells (added at 1 mL/well) at 104, 106, and 108 CFU/mL.
ME-180 cells were inoculated with both strains simultaneously, following which the cells were cultured for 5 h at 37°C in a 5% CO2 incubator. The culture medium was removed, and the ME-180 cells were washed twice with Dulbecco’s phosphate-buffered saline (DPBS; Welgene), followed by the addition of the culture medium (1 mL/well) and overnight incubation at 37°C in a 5% CO2 incubator. The next day, the culture medium was removed, and the cells were washed twice with DPBS, dehydrated, and subjected to Gram staining. The cells were stained with crystal violet for 1 min, washed twice with DPBS, decolorized with alcohol for 1 min, and washed twice with DPBS. Next, the cells were stained with Safranin O for 1 min, washed twice with DPBS, dehydrated, and examined under a microscope (Olympus CK2 inverted microscope, Olympus Corp., NY, USA).
ME-180 cells were also alternately inoculated with the two strains. First, the cells were inoculated with one of the two strains at 104, 106, and 108 CFU/mL, and the plate was incubated at 37°C in a 5% CO2 incubator for 5 h. The culture medium was removed, and the plate was washed twice with DPBS. Next, ME-180 cells were inoculated with the second strain at 106 and 108 CFU/mL and cultured at 37°C in a 5% CO2 incubator for 5 h. The culture medium was removed, and the ME-180 cells were washed twice with DPBS (1 mL/well), followed by overnight incubation with the basal medium at 37°C and 5% CO2. The ME-180 cells were stained as described above and examined under a microscope.
4. Results
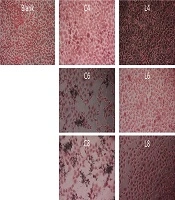
Figure 1 shows the results obtained upon the inoculation of ME-180 cells with L. plantarum and C. albicans. Inoculation with L. plantarum at 104, 106, and 108 CFU/mL did not affect the growth of ME-180 cells, nor did it cause any cell damage. Inoculation with C. albicans at 104 CFU/mL did not affect the growth of ME-180 cells. However, significant necrosis was observed in ME-180 cells inoculated with C. albicans at 106 and 108 CFU/mL. In contrast, low levels of apoptosis were observed in ME-180 cells simultaneously inoculated with 104 CFU/mL of C. albicans and L. plantarum at all culture concentrations. A higher level of apoptosis was observed in ME-180 cells inoculated with 106 CFU/mL of C. albicans, and the extent of apoptosis was not significantly affected by the concentration of the L. plantarum culture.
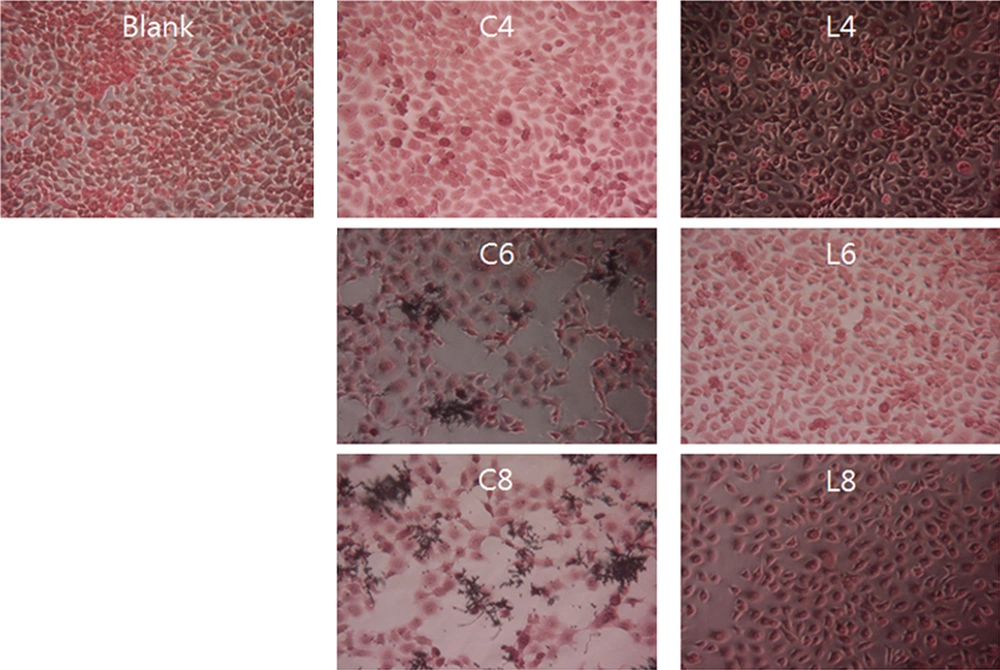
Extensive damage was observed in ME-180 cells inoculated with 108 CFU/mL of C. albicans, regardless of the concentration of the L. plantarum culture (Figure 2). The same results were obtained for ME-180 cells that were first inoculated with C. albicans and then with L. plantarum at different culture concentrations. Although inoculation with 104 CFU/mL of C. albicans did not inhibit the growth of ME-180 cells, low levels of cell damage were observed upon inoculation with 106 CFU/mL of C. albicans, and the growth of C. albicans was notable. There were no significant differences in the levels of ME-180 cell damage depending on the L. plantarum culture concentration. In contrast, ME-180 cells inoculated with 108 CFU/mL of C. albicans showed extensive cell damage, regardless of the L. plantarum culture concentration (Figure 3).
Morphology of ME-180 cells inoculated with Candida albicans and Lactobacillus plantarum. ME-180 cells were cultured with C. albicans for 5 h, then inoculated with L. plantarum, cultured for 5 h, washed, and cultured for an additional 24 h. C, C. albicans; L, L. plantarum. The numbers indicate 104, 106, and 108 CFU/mL, respectively.
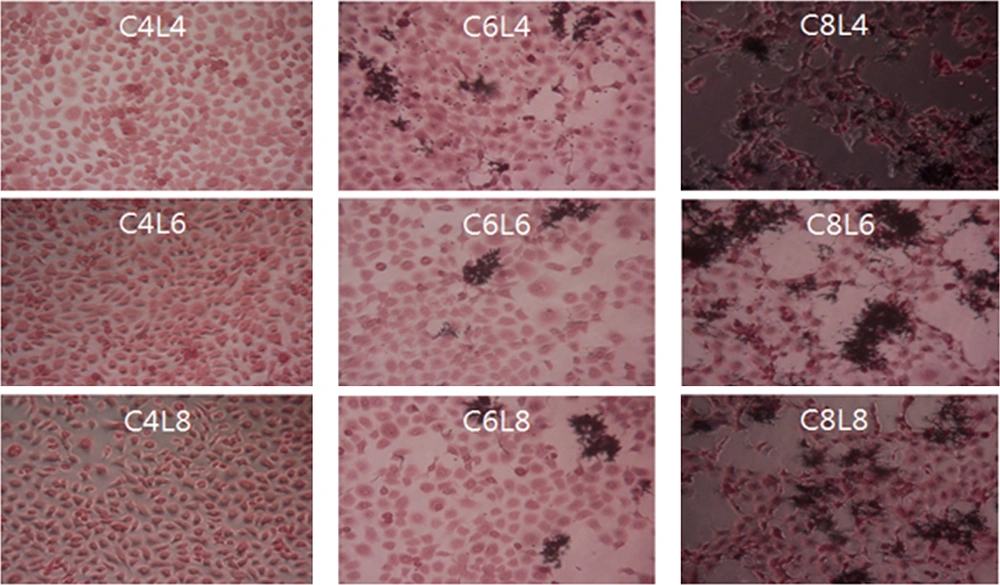
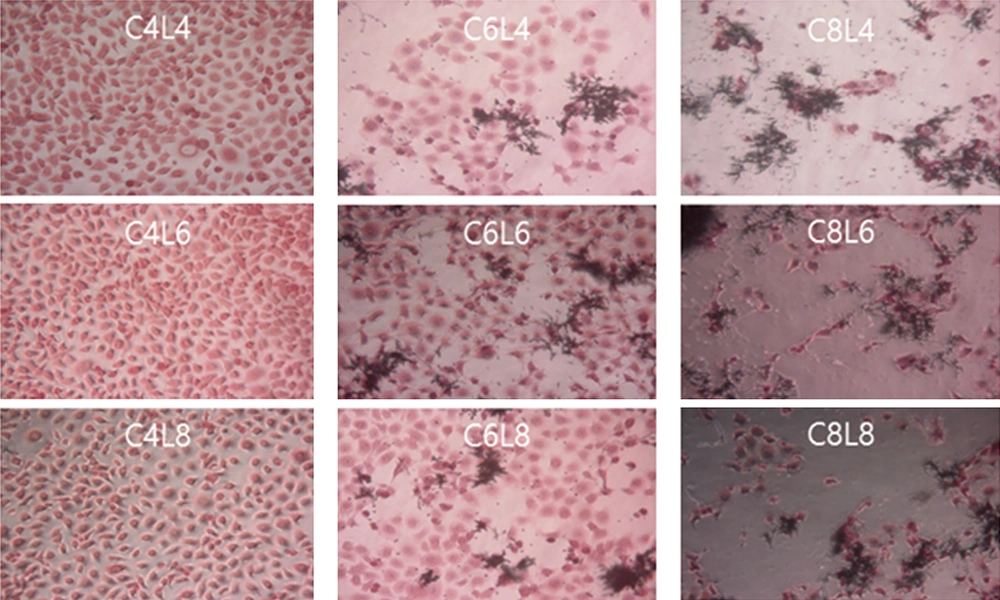
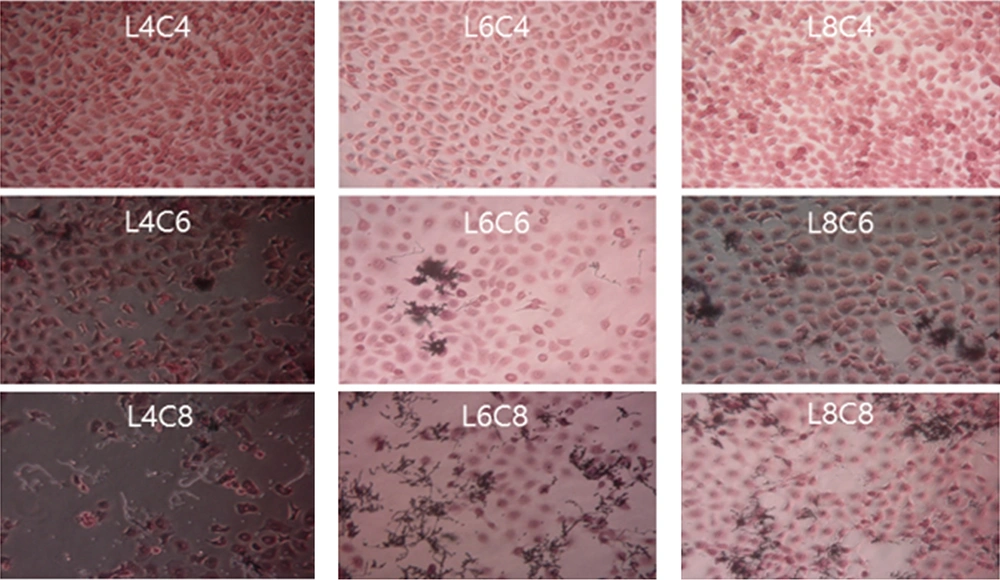
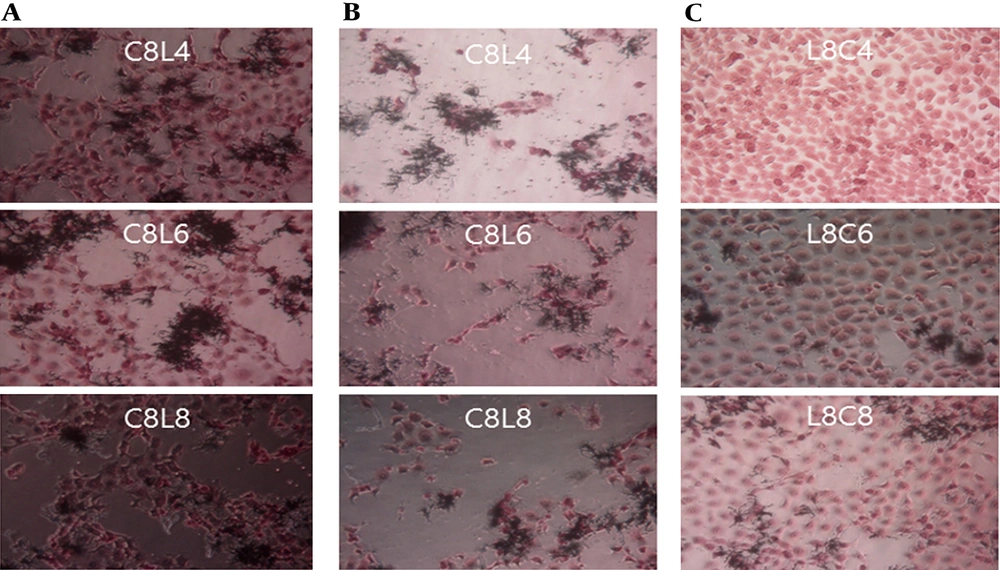
Different results were obtained when the ME-180 cells were first inoculated with L. plantarum and then with C. albicans at different culture concentrations, as shown in Figure 4. The extent of damage in ME-180 cells increased as the concentration of C. albicans increased in the cultures inoculated with 104 CFU/mL of L. plantarum. Low levels of cell damage were observed in the ME-180 cultures inoculated with 106 CFU/mL of L. plantarum, irrespective of the C. albicans culture concentration. Meanwhile, cell damage was noticeably reduced in the ME-180 cell cultures inoculated with 108 CFU/mL of L. plantarum, irrespective of the C. albicans culture concentration. Figure 5 shows the results for ME-180 cells simultaneously inoculated with 108 CFU/mL of C. albicans and 108 CFU/mL of L. plantarum, as well as for those inoculated with 108 CFU/mL of C. albicans or L. plantarum first, followed by inoculation with the other microorganism at the same concentration later. The extent of cell damage was the greatest in the second condition, followed by those in the first and third conditions.
Morphology of ME-180 cells inoculated with Candida albicans and Lactobacillus plantarum. ME-180 cells were cultured with L. plantarum for 5 h, then inoculated with C. albicans, cultured for 5 h, washed, and cultured for an additional 24 h. C, C. albicans; L, L. plantarum. The numbers indicate 104, 106, and 108 CFU/mL, respectively.
Antagonistic effects of Lactobacillus plantarum on Candida albicans in ME-180 cell cultures, depending on the order of inoculation. A. Simultaneous inoculation. B. Inoculation with C. albicans first. C. Inoculation with L. plantarum first. C, C. albicans; L, L. plantarum. The numbers indicate 104, 106, and 108 CFU/mL, respectively.
5. Discussion
Vaginitis is typically classified as bacterial vaginosis and candidal vaginitis. Candidal vaginitis, which is caused by C. albicans, is characterized by itching and the secretion of a thick, white vaginal fluid. An estimated 70 - 75% of women experience candidal vaginitis at least once in their lifetime, and 40 - 50% experience at least two recurrences within a year (15, 16). Santos et al. (17) reported that the secretion of anti-inflammatory cytokines and interleukin 8 and the activity of nuclear factor kappa B reduced in HeLa cervical carcinoma cells inoculated with L. plantarum and L. fermentum before and after inoculation with C. albicans. Kang et al. (18) reported that L. plantarum and L. fermentum attached to HT-29 human colorectal adenocarcinoma cells and inhibited the growth of C. albicans. Matsuda et al. (19) reported that L. gasseri and L. crispatus reduced the adhesion of C. albicans to HeLa cells.
In this study, L. plantarum was added to an ME-180 cell culture to examine its inhibitory effect on C. albicans. No damage was observed in the ME-180 cell cultures inoculated only with L. plantarum, whereas inoculation with C. albicans resulted in extensive damage to ME-180 cells. In contrast, the levels of damage to ME-180 cells increased significantly as the C. albicans concentration increased in ME-180 cultures simultaneously inoculated with L. plantarum and C. albicans, as well as in those that were first inoculated with C. albicans and then with L. plantarum. The same results were obtained when ME-180 cells were first inoculated with L. plantarum and then with C. albicans. However, the level of cell damage decreased noticeably in ME-180 cultures inoculated with L. plantarum at concentrations of 106 CFU/mL or higher, depending on the concentration of the C. albicans culture.
The inhibitory effect of L. plantarum on C. albicans in the ME-180 cell culture was not direct (e.g., via hydrogen peroxide, bacteriocin, lactic acid, and organic acids). Rather, the effect was presumed to be mediated by a reduction in the growth and mucosal adhesion ability of C. albicans by inhibition at the early stages of biofilm development. Lactobacillus plantarum, which exerted strong inhibitory effects on C. albicans, can be used as an auxiliary treatment agent for female vaginitis after safety studies have been conducted for assessing its metabolic activities and it has been confirmed to be non-infectious in animals.
5.1. Conclusions
The antagonistic effect of L. plantarum on C. albicans was observed in ME-180 cell cultures inoculated first with L. plantarum, and the effect increased as the concentration of the L. plantarum culture increased. However, almost no antagonistic effect was observed in ME-180 cell cultures simultaneously inoculated with L. plantarum and C. albicans, or in those inoculated with C. albicans first, regardless of the concentration of the L. plantarum culture. These findings indicate that L. plantarum may not have antagonized C. albicans directly by inhibiting its hydrogen peroxide, bacteriocin, lactic acid, and low pH. Rather, it may have inhibited biofilm development at an early stage, which subsequently inhibited the growth and mucosal adhesion ability of C. albicans in the ME-180 cell culture. Further studies should be conducted on the safety, side effects, and metabolism of L. plantarum, and it should be confirmed to be non-infectious in animals before the L. plantarum isolate derived from kimchi can be used as an auxiliary treatment agent for vaginitis.