1. Introduction
Nontuberculous mycobacteria (NTM) is an important pathogen, which can conditionally cause lung infection, skin infections, and central nervous system infections. According to the growth rate, NTM can be divided into slowly growing mycobacteria and rapidly growing mycobacteria (RGM). There is a considerable portion of RGM, including Mycobacterium abscessus, M. fortuitum, and M. chelonae that can induce diseases in human (1).
With the development of sequencing technology, M. mucogenicum group was identified as a novel type of NTM. Mycobacterium mucogenicum group included M. mucogenicum, M. aubagnense, and M. phocaicum. Mycobacterium mucogenicum, formerly named as M. chelonae-like organism, was firstly identified in 1982 and then recognized as a new species in 1995. Previous researches suggested that M. mucogenicum could be found in communal and nosocomial environments, including water body, soil, food, household, water system, and central venous catheter (2-4). As a conditional pathogen, M. mucogenicum rarely causes infections in clinical practice. Previous studies suggested that M. mucogenicum might infect immunocompromised patients, recent studies indicated that the risk of M. mucogenicum infection in immunocompetent patients was much higher than we thought (5). In consideration of the gloomy clinical outcome, it was necessary for clinicians and pharmacists to remain a high index of suspicion for M. mucogenicum (6). In the present study, we firstly reported a new case of M. mucogenicum and Klebsiella pneumoniae infection and discussed the pharmaceutical intervention and clinical management of M. mucogenicum infection.
2. Case Presentation
A 32-year-old non-smoking male patient was diagnosed with congenital atrial septal defect and severe pulmonary arterial hypertension for decades. The patient underwent cardiac surgery in the Affiliated Hospital of Guizhou Medical University. The initial hospital course of the patient was uneventful. Physical examination showed that the patient’s temperature was 36.6°C, heart rate (HR) was 74 beats/min, and blood pressure (BP) was 122/70 mmHg. Laboratory evaluation revealed the following findings: White blood cells (WBC) count was 9.96 × 109/L (93.20%, neutrophils), procalcitonin (PCT) was 1.46 ng/mL, and c-reactive protein (CRP) was greater than 20 mg/L on postoperative day 1 (POD 1). Clinicians and the clinical pharmacist thought that the increased WBC count, PCT, and CRP were resulted from cardiac surgery that might induce stress reaction. Cefuroxime (3.0 g/d, intravenous drip) was used to prevent infection. Although the ventricular premature beat was observed on POD 6, the patient’s medical conditions were well controlled. On POD 12, cough, blood in sputum, and fever (38.4°C) were observed in the patient.
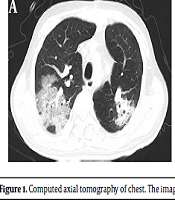
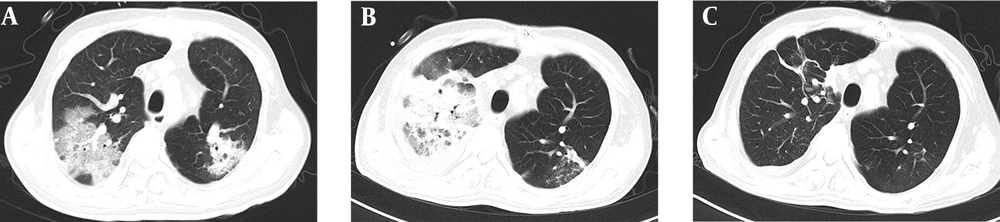
Laboratory evaluation consistently showed that WBC count was 15.54 × 109/L (86.10% neutrophils). Cefoperazone-sulbactam (9.0 g/d, intravenous drip) was administrated for the treatment of pulmonary infection. Unexpectedly, the patient rapidly developed dyspnea (SPO2 86%), which required mechanical ventilation on POD 15. Computerized tomography (CT) scan of the chest showed numerous plaque-like and ground glassy opacities in both lungs (see Figure 1A), which were similar to COVID-19 patterns. However, polymerase chain reaction (PCR) nucleic acid testing for COVID-19 was negative.
The antibiotic regimen, including cefoperazone-sulbactam (9.0 g/d, intravenous drip) and vancomycin (2.0 g/d, intravenous drip), was empirically used. Later, ceftazidime (6.0 g/d, intravenous drip) and carbapenem antibiotics were used. However, none of these antibiotics worked well. On POD 26 and POD 28, blood culture and sputum culture were positive for carbapenem-resistant K. pneumoniae, respectively. According to the susceptibility testing, the antibiotic was changed to tigecycline (100 mg/d, intravenous drip). Afterward, the patient’s temperature was decreased. Nonetheless, laboratory evaluation revealed that WBC count was 16.3× 109/L (87.70% neutrophils), PCT was 0.69 ng/mL, and interleukin-6 (IL-6) was 175.3 pg/mL, suggesting that the patient had developed sepsis.
The patient was readmitted to ICU on POD 33 for dyspnea with decreasing SPO2. Considering that the pulmonary infection might be caused by other Gram-positive bacilli and (or) fungal infections, an antibiotic regimen consisting of ceftazidime-avibactam (7.5 g/d, intravenous drip), vancomycin (2.0 g/d, intravenous drip), and micafungin (400 mg/d, intravenous drip) was added. Unfortunately, the patient developed acute kidney failure (urinary volume: 1300 mL; Urea: 14.59 mM; creatinine: 126.0 μM) on POD 37. We believed that acute kidney failure might result from the adverse drug reaction of vancomycin and (or) sepsis. Therefore, we discontinued using vancomycin and began continuous renal replacement therapy (CRRT) for this patient.
On POD 39, the bronchoscopies with bronchoalveolar lavage were performed on the patient. Further, the identification of the pathogen in bronchoalveolar lavage fluid was performed by next-generation sequencing (PMSEQ, Beijing Genomics Institute). The sequencing result identified that M. mucogenicum and K. pneumoniae were the potential pathogens in bronchoalveolar lavage fluid. According to the previous studies, clarithromycin 0.25 g orally every 8 hours was administrated for a total of 32 days, together with linezolid 600 mg orally twice daily for 53 days. Additionally, tigecycline (100 mg/d, intravenous drip) and ceftazidime-avibactam (3.75 g/d, intravenous drip) were also used for the treatment of K. pneumoniae infection. Following these treatments, the medical condition of the patient substantially improved, the mechanical ventilation and CRRT were stopped on POD 50 and POD 59, respectively. There was no evidence of relapsed infection after discontinuation with antibiotics (see Figure 1B and C).
3. Discussion
More than 100 species of RGM have been identified and traditionally classified into M. abscessus complex, M. chelonae, M. fortuitum complex, and M. mucogenicum. Mycobacterium mucogenicum, as one of the most common type of RGM, can produce mature colonies on culture media within 7 days. In the past years, M. mucogenicum was considered to be a conditional pathogen. However, recent studies demonstrated that the incidence of M. mucogenicum infection was increased worldwide, which might result from the progression of pathogen identification technology and sequence technology (5). In the present work, we firstly reported a new case of M. mucogenicum and K. pneumoniae pulmonary infection in a patient after cardiac surgery.
Mycobacterium mucogenicum was firstly misidentified as M. chelonae-like organism in 1982, but the result of 16S rRNA sequence indicated it was more closely to M. fortuitum in phylogenetically system. Finally, it was named M. mucogenicum due to the mucoid-like cell surface by which grew on solid media. The characteristic mucoid surface could promote the resistance of M. mucogenicum to chlorine in water and the ability to form biofilms, which might explain why M. mucogenicum mainly caused infection through contaminated materials, waters, and invasive operations (7, 8).
In the past, the pathogenic properties of M. mucogenicum was principally in immunocompromised hosts. However, increasing studies had identified that M. mucogenicum also could infect immunocompetent patients (6). Although there was no identifiable risk factor for pulmonary infection caused by RGM, the previous study indicated that there were several predisposing factors for environmental mycobacteria-related lung diseases, including structural lung diseases, decreased clearance of sputum, gastroesophageal reflux disease, endocrine or immune, prior lung infections, slender body habitus, and so on (9). The present patient is 1.55 meters tall and weighs 48 kilograms, which may promote the risk of M. mucogenicum infection. Additionally, the patient was diagnosed with congenital heart defect, pulmonary arterial hypertension, mitral regurgitation, and tricuspid regurgitation, which might also increase the risk of pulmonary infection. We believed that these host conditions might promote the risk of M. mucogenicum infection.
This patient quickly developed acute respiratory distress syndrome after showing symptoms of lung infection, which brought great challenges. Rapid and reliable identification of pathogens is crucial to the treatment of M. mucogenicum. The diagnosis of M. mucogenicum needs a comprehensive understanding of the pathogens of the organism. Additionally, applicable methods for bacterial culture are vital for the identification of M. mucogenicum. However, several times of blood culture and sputum culture in our case were negative for M. mucogenicum that might postpone the treatment of the patient. Finally, next-generation sequencing, which was thought the gold standard for M. mucogenicum identification, revealed that the M. mucogenicum and K. pneumoniae were the true pathogens in the patient.
Once diagnose was determined, the appropriate antibiotics should be taken. The prolonged and combination antimicrobial treatment is the characteristic of M. mucogenicum management. Previous studies indicated that M. mucogenicum was susceptible to aminoglycosides, clarithromycin, quinolones, and trimethoprim-sulfamethoxazole (4, 10). However, the acute renal failure of the patient limited our choice. In consideration of the M. mucogenicum and K. pneumoniae infections, we decided to use clarithromycin, linezolid, tigecycline, and ceftazidime-avibactam. In addition, this antibiotic regimen has been used for more than four weeks and successfully treated the patient.
3.1. Conclusions
Mycobacterium mucogenicum may result in severe pulmonary infections, especially co-infection with other pathogens. Pathogen sequence technology is considered a gold standard for identifying M. mucogenicum. Additionally, clinicians and the clinical pharmacist should be aware of other possible copathogens in dealing with M. mucogenicum infection.