1. Background
Tuberculosis is an infectious disease and is a leading cause of death. According to the latest WHO report, 10 million new tuberculosis cases in 2019, including 1.2 million deaths, were attributed to tuberculosis (1). This increased incidence of tuberculosis cases is due to the multidrug-resistant, or at least rifampicin (RIF)- or isoniazid (INH)-resistant, multidrug resistant Mycobacterium tuberculosis (MDR- tuberculosis) strains, becoming a threat to tuberculosis control programs (2, 3). Early detection of resistance against first-line drugs, namely INH, RIF, ethambutol (EMB), streptomycin (STR) and pyrazinamide (PZA), is essential to control the spread of MDR- tuberculosis strains (4, 5). Moreover, the increased emergence of MDR- tuberculosis is a healthcare threat that has been reported (6, 7). Thus, there is an urgent need for rapid, cost-effective, and reliable methods for diagnosis and drug susceptibility testing in tuberculosis, especially in low-resource settings (8, 9).
The conventional methods for drug susceptibility testing of M. tuberculosis include time-consuming methods, such as the proportion method using Löwenstein Jensen medium or Middlebrook 7H10 agar and those involving heavily-mechanised equipment and radioactive materials such as the BACTEC TB-460 system (Becton Dickinson) (10). Recently, several methods have been proposed for the rapid diagnosis and drug susceptibility testing of tuberculosis (10, 11). Among these methods, the Mycobacterial Growth Indicator Tube (MGIT, Becton Dickinson) and molecular tools, such as the INNO-LiPA Rif. Tuberculosis (Line probe assay, Innogenetics, Ghent, Belgium) and Gene Xpert MTB/RIF (Cephid, USA), have been used extensively. These methods are expensive and impractical for routine use (10).
Thin-layer agar is a fast test for the diagnosis and drug susceptibility testing of pulmonary and extrapulmonary tuberculosis (12, 13). This technique has been described previously as a cost-effective method, and it provides rapid results compared to conventional methods (14). Direct thin-layer agar can be used for simultaneous detection of M. tuberculosis and drug susceptibility testing (15), avoiding the intermediate step of M. tuberculosis isolation that reduces the time and need for a high level of biohazard containment (16). These features make thin-layer agar a convenient method for low-resource settings (17). A limited number of studies have evaluated the performance and applicability of thin-layer agar in routine practice (14).
2. Objectives
The primary aim of this study was to evaluate the performance of thin-layer agar in the fast detection of M. tuberculosis and drug susceptibility testing as first-line anti- tuberculosis agents, applied to different sample types. The results were evaluated and compared with the reference standard.
3. Methods
3.1. Study Setting
This cross-sectional prospective study was conducted between May 2018 and October 2020 at Abbassaia Chest Hospital and Ain Shams University Hospital, Cairo, Egypt. Inclusion criteria were patients with clinical signs of tuberculosis. Exclusion criteria patients who started tuberculosis treatment for more than one week.
3.2. Specimen Collection and Processing
Sputum, stool, and body fluid samples were collected from patients with suspected tuberculosis. Samples were contained in new plastic containers prior to treatment. All collected samples were stored at 4°C and analysed within 24 h. Sputum samples were decontaminated with N-acetyl-L-cysteine sodium hydroxide and concentrated via centrifugation (3,000× g) for 30 min, followed by acid fast bacilli (AFB) microscopy and cultivation. Body fluid and abscess aspirates were collected under sterile conditions and used directly for AFB microscopy and cultivation.
3.3. Routine Laboratory Testing
The presence of AFB was noted and quantified using AFB staining (18). Both smear-positive and smear-negative samples were cultured using Lowenstein Jensen culture medium (Oxoid, Hampshire, United Kingdom), and drug susceptibility testing of isolates was performed using proportion method on 7H10 Middlebrook agar (Difco Laboratories, Detroit, USA), which was used as medium for the first-line drug according to the Clinical and Laboratory Standards Institute (CLSI) procedures and recommended critical concentrations (19).
3.4. Thin-Layer Agar Test
A 5 mL of 7H11 Middlebrook agar (Difco Laboratories) were poured onto Petri plates that are divided into quadrants. Per quadrant contains the following: growth control with no additions, with a quadrant containing a specific inhibitor of M. tuberculosis complex (0.5 mg/mL of para-nitrobenzoic acid), a quadrant with 0.2 μg/mL INH, and a quadrant with 1 μg/mL of RIF. Other plates were prepared with 2 μg/mL of STR and 75 μg/mL EMB (20). Samples were decontaminated and diluted according to the AFB load on the smear as follows: > 250 AFB/field, 10−3 dilution, 25 - 250 AFB/field, 10−1 dilution, < 25 AFB/field, no dilution, while smear-negative samples were not diluted. Each plate quadrant was inoculated with 0.1 mL of diluted sample, tape-sealed, and incubated at 37°C in a 5% CO2 incubator. Plates were observed using a conventional microscope at 100× magnification every two days over one month. The specimen was considered positive for M. tuberculosis when the growth was recorded in the control quadrant and resistant if growth was observed in quadrants containing RIF, INH, STR, and EMB as compared to the growth control. All results were read blindly to the reference tests.
3.5. Data Analysis
Receiver operating characteristic curve analysis was performed using MedCalc version 11.61 (MedCalc Software, Mariakerke, Belgium), and sensitivity and specificity were calculated for detection of M. tuberculosis and drug susceptibility testing by thin-layer agar. Key proportions were reported with 95% confidence (95% Confidence Interval (CI)), and a P-value of < 0.05 was considered statistically significant. The time for M. tuberculosis detection was recorded, and the time for drug susceptibility testing was calculated from sample processing until the appearance of sufficient growth to read the samples from thin-layer agar, Lowenstein Jensen culture method, and culture proportion method. The time was compared using Microsoft Excel version 365 (Microsoft, USA) for both thin-layer agar and conventional methods, and the uninterpretable samples were excluded.
4. Results
During the study period, 241 samples were collected, in which 2 samples were excluded, and 239 samples were eligible. Of the 239 samples, 123 were smear positive (105 were sputum, 12 body fluid, and 6 pus) and 116 were smear negative (107 were sputum and 9 body fluid). Detection of M. tuberculosis using thin-layer agar showed a 93.7% agreement between thin-layer agar and Lowenstein Jensen culture method (Table 1). Detection of M. tuberculosis using thin-layer agar showed a sensitivity of 96.63% and specificity of 93.50%, and the CI values were at 0.910 - 0.966 (95% CI) when compared to Lowenstein Jensen culture (Table 2). Drug susceptibility testing using thin-layer agar showed that the sensitivity level of specimens against INH, RIF, EMB, and STR were 94.74%, 86.84%, 94.74%, and 81.58%, respectively. Specificity levels of drug susceptibility testing using thin-layer agar were 100%, 100%, 86.27%, and 100% for INH, RIF, EMB, and STR, respectively (Table 2). The area under the curve (AUC) for detection of M. tuberculosis was 0.916, and drug susceptibility testing were 0.974, 0.934, 0.905, and 0.908 for INH, RIF, EMB, and STR, respectively (Table 2).
| Test | Sensitivity | Specificity | 95% CI | AUC |
|---|---|---|---|---|
| Mycobacterium tuberculosis detection | 96.63 | 93.50 | 0.910 - 0.966 | 0.916 |
| Susceptibility testing | ||||
| Isoniazid | 94.74 | 100.00 | 0.915 - 0.996 | 0.974 |
| Streptomycin | 81.58 | 100.00 | 0.828 - 0.959 | 0.908 |
| Ethambutol | 94.74 | 86.27 | 0.824 - 0.957 | 0.905 |
| Rifampicin | 86.84 | 100.00 | 0.861 - 0.976 | 0.934 |
Specimen-Wide Distribution of Mycobacterium tuberculosis Isolates
| Specimen | No. of Samples | Both +ve | Both -ve | LJ +ve, TLA -ve | LJ –ve, TLA +ve | Agreement (%) |
|---|---|---|---|---|---|---|
| Sputum | 212 | 71 | 129 | 1 | 4 | 96.7 |
| Body fluid | 21 | 7 | 12 | 0 | 2 | 90.5 |
| Pus | 6 | 2 | 3 | 0 | 1 | 83.3 |
| Total | 239 | 80 | 144 | 1 | 7 | 93.7 |
Mycobacterium tuberculosis Susceptibility Testing Against First Line Anti-tuberculosis Drugs Using Lowenstein Jensen Culture and Thin-Layer Agar Methods.
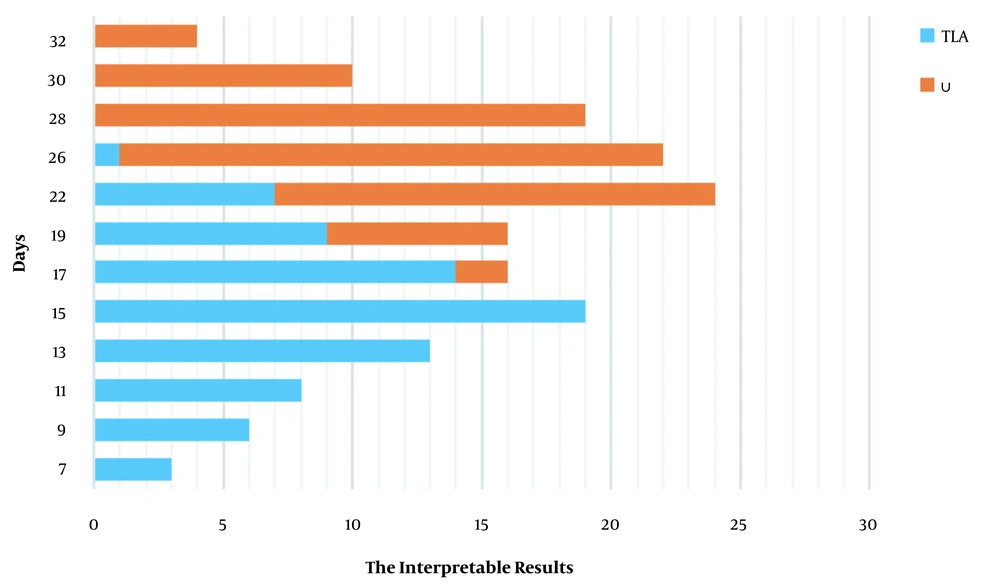
The median time for drug susceptibility testing from sample processing to readable results was 16 days, which was significantly faster than Lowenstein Jensen culture method - proportion method that had a median of 29 days (P < 0.01) (Figure 1).
5. Discussion
This study investigated the performance of thin-layer agar to detect M. tuberculosis and drug susceptibility testing to the first-line anti- tuberculosis agents. Our study reported a high positivity rate of M. tuberculosis detection because of the fast processing of the samples directly after collection and decontamination. Moreover, we showed a high positivity frequency, which may be due to selection bias, and the inclusion criteria were highly suspected cases of tuberculosis. Delay in testing of the specimens affects the positive results and increases the chance of contamination; thus, thin-layer agar is a suitable method for peripheral laboratories (21). Studies conducted in Switzerland showed a high positivity rate in the detection of M. tuberculosis using thin-layer agar in low-resource settings (21, 22). More M. tuberculosis -positive cultures were detected using thin-layer agar and MGIT than with Lowenstein Jensen culture method. Thin-layer agar and MGIT had a shorter detection time for M. tuberculosis growth (median 10 and 7.1 days, respectively) than Lowenstein Jensen culture method (median 22 days) and specimen bacillary load (23).
Finding a rapid, reliable, and cost-effective diagnostic method will be a key role in tuberculosis control, especially in low-resource settings. In our study, the median time for M. tuberculosis diagnosis and drug susceptibility testing using thin-layer agar was 16 days as compared when using Lowenstein Jensen culture method and proportion method (29 days). In a multi-centre study, BACTEC took approximately 11 days for direct drug susceptibility testing (24, 25). Although BACTEC takes a shorter time, we must consider that it is not available in low-resource settings. Molecular techniques provide drug susceptibility testing on the same day (26). Although molecular techniques are superior, their high cost and required level of training make them impractical in peripheral laboratories and low resource settings. In our study, using microscopic observations in thin-layer agar reduced the time compared to macroscopic detection. Supporting findings were reported by Welch et al. median detection time using thin-layer agar. M7H11medium was reported as 10 days, and the time was shorter in the case of smear-positive samples (27, 28).
In the current study, tuberculosis diagnosis using thin-layer agar showed a sensitivity of 96.63% and specificity of 93.5%. In concordance with our results, a recent study in Estonia found that thin-layer agar showed a sensitivity and specificity of 93.0% 99.4%, respectively, versus 62.5% and 99.3% for Xpert®MTB/RIF (16). Our results on the sensitivity and specifity rates on drug susceptibility testing using thin-layer agar were similar with Robledo et al., (23) wherein thin-layer agar showed 100% sensitivity and specificity for the RIF and INH resistance (23). Several studies used redox along with thin-layer agar. This method reduced the time, and the performance results were consistent with our findings. The coloured thin-layer agar test showed a sensitivity of 94.7% for levofloxacin, 95.8% for INH, and 97.3% for RMP. All tested drug specificities were > 97% (29). In Ethiopia, the colour test reported a sensitivity of 59%, 96%, and 95% for INH, RMP, and MDR- tuberculosis, respectively. Furthermore, it detected a specificity of 96%, 94%, and 98%, for INH, RMP, and MDR- tuberculosis, respectively (30).
Study limitations. This study aimed to evaluate the performance of thin-layer agar for the detection of M. tuberculosis and drug susceptibility testing and its implementation in low-resource settings. The high positivity rate in our study was due to selection bias and did not reflect disease prevalence. In this study, sensitivity calculations considered only patients with confirmed tuberculosis. The results of thin-layer agar were close to those of AFB, implying that a high positive rate would enhance thin-layer agar sensitivity. Only four smear-negative samples were positive using thin-layer agar, and this was not sufficient to evaluate its performance in drug susceptibility testing in smear-negative cases; thus, further studies are required. Furthermore, the study did not examine the performance of thin-layer agar for the phenotypic detection of new mutations that may result from inadequate treatment.
5.1. Conclusions
In conclusion, these results show that the thin-layer agar method is a rapid, simple, and cost-effective tool for the detection of M. tuberculosis and drug susceptibility testing. Our findings provide an approach to use thin-layer agar in low-resource settings and peripheral labs. Further studies are required for the large-scale evaluation of thin-layer agar and its ability to detect new mutations in M. tuberculosis and extensive drug resistance M. tuberculosis.