1. Background
Staphylococcus aureus is a highly virulent pathogen, which is considered a critical threat to human health. It can cause different forms of infections at different sites, including the skin and soft tissue, bone, and joint. Furthermore, the bacteria can be seeded into the bloodstream from an initial lesion, resulting in S. aureus bacteremia, a mortal condition (1). Staphylococcus aureus bacteremia has two origins, community-acquired infection [CAI] and healthcare-associated infection [HCAI]), and its overall incidence is reported at about 10 to 30 per 100,000 person-years (2), varying according to the pathogen’s genotype (3). The considerable issue about S. aureus bacteremia is its high mortality rate (about 10% - 30%), which exceeds the mortality rate of other prevalent infectious diseases, such as acquired immunodeficiency syndrome, tuberculosis, and viral hepatitis combined (4).
Despite the significant drop in the mortality rate of S. aureus bacteremia since the introduction of antibiotics, their overuse caused the resistance of this pathogen to antibiotics, resulting in an increasing trend in the incidence of S. aureus bacteremia in recent decades in many countries (5). One of the most important antibiotic resistance of S. aureus is the methicillin-resistant S. aureus (MRSA), caused by mecA gene mutation that encodes an altered penicillin-binding protein (6). Methicillin-resistant S. aureus, a prominent cause of multidrug-resistant organism (MDRO), was initially observed in HCAIs, while it extended to CAIs, as well, which results in an increased mortality rate of S. aureus bacteremia (7).
Research on predictors of mortality has shown that cases with a catheter-related infection, persistent fever after 72 hours, abnormal findings on transesophageal echocardiogram, implanted prosthetic material, and metastatic infection are considered complicated and require prolonged treatment (4 - 6 weeks) (8). Infective endocarditis is another important complication of S. aureus bacteremia that can increase the risk of mortality and call for additional treatments, such as valve replacement surgery (9). Other risk factors and complications of S. aureus bacteremia associated with mortality and prolonged treatment include age, underlying conditions, admission to the intensive care unit, injecting drug abuse, and intravascular catheters or prosthetic devices (10, 11).
A review of Iranian studies (published from 2005 to 2012) indicated a high rate of MRSA in Iran, varying from 20.5% in Isfahan to 90% in Tehran with positive mecA gene in 52.7% of cases (12). Another review on Iranian publications from 2000 - 2017 showed 25.2% MRSA in strains with the capability of producing toxic shock syndrome (13). However, there is little information on the recent status of MRSA bloodstream infection (incidence, mortality, and associated factors) in Iran, and recent studies have only reported results of small populations, mainly outpatients, from different districts of the country (14-16). Therefore, there is a need for investigation of new information about MRSA in inpatients, the in-hospital mortality rates, and associated factors.
2. Objectives
Because of the expanding importance of S. aureus bacteremia, especially MRSA, and because of the differences in the epidemiological pattern of bacteremia in different geographical locations, we decided to investigate this problem in two hospitals of Northeast of Iran to indicate the factors associated with MRSA, duration of hospital admission, intensive care unit admission, infective endocarditis, and in-hospital mortality.
3. Methods
This cross-sectional study was conducted in two major general hospitals of Mashhad, Northeast of Iran, Imam Reza and Ghaem Hospitals, and the hospital information system was used as the source of data collection. The medical records of patients who were referred during March 2018 - 2019 were evaluated, and the patients older than 15 years old, who had at least one positive result of blood culture for S. aureus were included in the study. The medical records of 140 patients with S. aureus bacteremia were evaluated. Thirteen patients were younger than 15 years old and not enrolled. Those with contaminated cultures (n = 2) and incomplete medical records (n = 7) were not included in the study, as well. Accordingly, 104 patients were found eligible and considered as the final study population of the study. One patient was admitted twice during the study period (with a 10-month interval) and had S. aureus bacteremia in both admissions. As each admission of this patient was considered separately, the total number of S. aureus bacteremia evaluated reached 105 cases.
The blood samples were taken by an experienced nurse or blood sampler, approved by the hospitals’ managers, under sterile conditions and according to the Clinical and Laboratory Standards Institute (CLSI) standards. For this purpose, the samples were collected in BACTECTM blood culture system and some in traditional cultures and sent to the microbiology laboratory within one hour, where they were cultured in chocolate agar, blood agar, and eosin methylene blue for 72 hours. Non-contaminated samples, of which only S. aureus was cultured, were considered positive. Staphylococcus aureus bacteremia was defined as isolation of the organism from at least one blood sample. The positive samples were evaluated by additional microscopic examinations; after staining, the morphological characteristics of the organism were evaluated. Methicillin-resistant S. aureus was confirmed by the presence of strains’ resistance to oxacillin or cefoxitin in the disc diffusion method. All stages of preparation and storage of samples, as well as culture and identification of antibiotic-resistant species, were performed according to CLSI.
Bacteremia was considered “hospital-acquired infection (HAI)” if the first positive blood culture was obtained 48 hours after admission. Staphylococcus aureus bloodstream infection (SABSI) was defined as isolation of S. aureus during the first 48 hours of admission, which can be either HCAI or CAI. Positive blood cultures, with one or more of the below criteria, were considered “HCAI”: (1) Residence in a health care or nursing facility; (2) Taking outpatient hemodialysis; (3) Being hospitalized for ≥ 2 days within 90 days of current admission; and (4) Receiving intravenous antibiotics or chemotherapy or wound care within 30 days (17).
Systemic inflammatory response syndrome (SIRS) was defined as having at least 2 of the following signs/symptoms: a central body temperature of ≥ 38°C or ≤ 36°C, heart rate of ≥ 90 beats/min, respiratory rate of ≥ 20 per min or partial pressure of carbon dioxide (PaCO2) ≤ 32 mmHg, white blood cell of ≥ 12,000 or ≤ 4,000 in m3 or ≥ 10% band cell; cases with SIRS plus positive results of blood culture were considered sepsis. In this study, the practical definition of infective endocarditis was considered probable and definite endocarditis, according to duke criteria (18), which considers two major criteria: At least two positive blood cultures from blood samples taken > 12 hours apart (for organisms that are typical causes of endocarditis) or ≥ 3 separate blood cultures (first and last at least one hour apart, for organisms that are more commonly skin contaminants), and echocardiographic evidence or new heart murmur indicating heart valve regurgitation and five minor criteria: (1) Predisposing factors, like intravenous drug abuse, positive history of infective endocarditis, rheumatic heart disease, valvular disease, cyanotic diseases, hypertrophic cardiomyopathy, prosthetic cardiac devices, and injection drug abuser; (2) vascular events, such as major arterial emboli, infectious pulmonary infarcts, mycotic aneurysm, intra-cerebellar hemorrhage, conjunctival hemorrhage, Janeway lesions; (3) immunological events, such as Osler node, glomerulonephritis, Roth spots, and rheumatoid factor; (4) fever ≥ 38°C; and (5) microbiological evidence, like cultures not included in the major criteria or serological evidence of Q fever. Cases with two major criteria, or one major and three minor, or all of the five minor criteria were considered definite infective endocarditis, and cases with one major and one minor criterion or three minor criteria were considered probable of infective endocarditis.
The demographic characteristics of the patients such as age and sex, as well as clinical data, such as duration of admission, positive history of underlying diseases (including neurologic, rheumatologic, endocrine, and cardiovascular diseases, and neoplasms), and recent use of antibiotics (during the past one month before admission), were recorded. The results of physical examinations and diagnostic criteria of SIRS and sepsis were recorded, as well. The interval between admission and blood culture sampling was recorded. The results of positive cultures from other sites were recorded, which included central venous line, bedsore, and surgical site, bronchial secretions, synovial fluid, and pleural fluid. The main outcomes of the present study included the MRSA results, infective endocarditis, source of infection (CAI or HCAI), the final outcome of the patients (considered as “discharge with good conditions” and “death”), and intensive care unit requirement. The cases who left the hospital with their own consent before diagnosis of the foci were also recorded.
We used one-sample Kolmogorov-Smirnov test to investigate the normal distribution of the data. The parametric data were described using mean ± standard deviation (SD) and compared between the groups using independent t-test or one-way ANOVA; non-parametric data were described using median (minimum-maximum) and compared between the groups using Mann-Whitney U-test. The categorical variables were described using number (percentage) and compared using the chi-square test. The statistical analysis was performed using SPSS for Windows version 16.0. (SPSS Inc. Released 2007. Chicago) and P-values of less than 0.05 were considered statistically significant.
4. Results
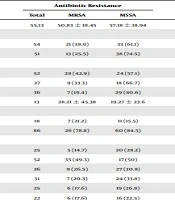
A total of 105 cases were included in the statistical analysis; fifty-four cases were male (51%); the male-to-female ratio was 54/51. The mean age of the study population was 55.13 years (15 - 88 years). The mean age of patients was not different between males and females (53.6 ± 17.6 vs. 56.4 ± 20.4; P = 0.443). The frequency of neurologic, endocrine, and cardiovascular diseases, and neoplasms was 65.8% (n = 69), 92.4% (n = 97), 76.2% (n = 80), and 79% (n = 83), respectively. The frequency of underlying diseases is shown in Table 1. Only three patients had no history of underlying diseases and used no medications. Of all patients, 21.2% had foreign object of any kind (like prosthesis, drain, and central venous line), and 17.3% had recent use of antibiotics during the past one month before admission. The median duration of hospital admission was 13 days, neither different based on the patients’ age (P = 0.101), nor sex (mean of 26.6 days in women and 17.2 days in men, P = 0.413; based on the results of Mann Whitney U-test). In 15 cases, S. aureus was also isolated from other sites: nine samples from central venous line, six samples from bedsores, and surgical sites.
| Variable | Antibiotic Resistance | P-Value | ||
|---|---|---|---|---|
| Total | MRSA | MSSA | ||
| Age, y | 55.13 | 50.83 ± 18.45 | 57.18 ± 18.94 | 0.86* |
| Sex | 0.143† | |||
| Male | 54 | 21 (38.9) | 33 (61.1) | |
| Female | 51 | 13 (25.5) | 38 (74.5) | |
| Source of infection | 0.029† | |||
| Hospital | 52 | 28 (42.9) | 24 (57.1) | |
| Community | 27 | 9 (33.3) | 18 (66.7) | |
| Healthcare | 36 | 7 (19.4) | 29 (80.6) | |
| Duration of hospitalization, d | 13 | 28.21 ± 45.38 | 19.27 ± 23.6 | 0.365 |
| Recent antibiotic use | 0.252† | |||
| Yes | 18 | 7 (21.2) | 11 (15.5) | |
| No | 86 | 26 (78.8) | 60 (84.5) | |
| Underlying diseases | ||||
| Cardiovascular diseases | 25 | 5 (14.7) | 20 (28.2) | 0.130† |
| Hypertension | 52 | 35 (49.3) | 17 (50) | 0.946† |
| Neurological diseases | 36 | 9 (26.5) | 27 (30.8) | 0.243† |
| End-stage renal disease | 31 | 7 (20.3) | 24 (33.8) | 0.165† |
| Immunocompromised | 25 | 6 (17.6) | 19 (26.8) | 0.305† |
| Neoplasm | 22 | 6 (17.6) | 16 (22.5) | 0.565† |
| Patient’s outcome | 0.614† | |||
| Discharge | 56 | 21 (61.8) | 35 (56.5) | |
| Death | 40 | 13 (38.2) | 27 (43.5) | |
| Central venous line infection | 0.571† | |||
| Yes | 38 | 11 (28.9) | 27 (71.1) | |
| No | 67 | 23 (34.3) | 44 (65.7) | |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
aValues are expressed as No. (%) or mean ± SD.
b*, The results of independent samples t-test; †, the result of chi-square test; ‡, the result of Mann-Whitney U-test.
The first blood sample, resulting in a positive culture, was taken during the first 48 hours in 60% of the patients (n = 62), and the mean duration of admission until the first positive blood sample was 8.25 days (maximum of 101 days). Thirty-four patients (32%) had MRSA in at least one blood sample. The association of study variables with the results of antibiotic resistance is shown in Table 1. As demonstrated, antibiotic resistance was only different based on the source of infection (P = 0.029), while other variables, such as patients’ mean age, sex distribution, recent antibiotic use, underlying diseases, and patients’ outcome, were not different between MRSA and methicillin-sensitive S. aureus (MSSA) cases (P > 0.05; Table 1). As demonstrated in this table, most cases of HAI, CAI, and HCAI had MSSA, and among all cases of MRSA, 52.9% were HAI, 26.5% were CAI, and 20.6% were HCAI (P = 0.029; Table 1). Forty-two patients had HAI, 36 patients (34%) had HCAI, and 27 patients (26%) had CAI. The distribution of patients’ sex and the outcome was not different based on the source of infection (P > 0.05; Table 2); but the mean duration of hospital admission was different according to the source of infection (P < 0.001); 37.26 ± 42.64 days in hospital-acquired infection, 14.5 ± 13.7 days in community-acquired infection, and 9 ± 10.6 days in HCAI.
| Variable | Source of Infection | P-Value | ||
|---|---|---|---|---|
| Hospital | Community | Healthcare | ||
| Sex | 0.626* | |||
| Male | 20 (37) | 16 (29.6) | 18 (33.3) | |
| Female | 22 (43.1) | 11 (21.6) | 18 (35.3) | |
| Duration of hospitalization, d | 37.26 ± 42.64 | 14.5 ± 13.7 | 9 ± 10.6 | < 0.001† |
| Intensive care unit admission | - | |||
| Yes | 8 (50) | 4 (25) | 4 (25) | |
| No | 34 (81) | 23 (85.2) | 32 (88.9) | |
| Antibiotic resistance | 0.029* | |||
| MSSA | 24 (57.1) | 18 (66.7) | 29 (80.6) | |
| MRSA | 28 (42.9) | 9 (33.3) | 7 (19.4) | |
| Patient’s outcome | 0.654* | |||
| Discharge | 24 (51.7) | 18 (69.2) | 14 (50) | |
| Death | 18 (42.9) | 8 (30.8) | 14 (50) | |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
aValues are expressed as No. (%) or mean ± SD.
b*, The result of chi-square test; †, the result of Mann-Whitney U-test.
Twenty-eight patients had infective endocarditis (3 definite and 25 probable infective endocarditis). The association of the study variables with the presence of infective endocarditis is shown in Table 3. As indicated, the mean age of patients and duration of hospital admission were neither different between the patients with and without infective endocarditis, nor was the frequency of antibiotic resistance, patients' outcome, and the presence of SIRS at admission (P > 0.05; Table 3).
Among the underlying diseases, the frequency of neurological diseases (53.6% vs. 27.3%; P = 0.001) and end-stage renal disease (60.7% vs. 18%; P = 0.001) were higher in patients without infective endocarditis (Table 3). However, the frequency of the source of infection, prosthetics, considerable foci of infection, and receipt of blood and its derivatives were significantly different between patients with and without infective endocarditis (P < 0.05; Table 3). Finally, 65 patients (61.9%) were discharged with good condition, 40 patients (38.1%) died, and nine patients (9%) left the hospital before a before diagnosis of the foci. The sex distribution of patients and source of infection were not different among the three categories (Table 4). The mean duration of blood sampling until death in cases that passed away was 7.57 ± 9.46 days (median of 2 days). Sixteen patients (15%) required intensive care unit admission. The mean age of the patients was not different between patients with or without intensive care unit admission (56 ± 18.9 vs. 48 ± 18.6; P = 0.127).
| Variable | Infective Endocarditis | P-Value | |
|---|---|---|---|
| Yes | No | ||
| Age, y | 57.46 ± 17.33 | 54.08 ± 19.56 | 0.397* |
| Source of infection | 0.038† | ||
| Hospital | 7 (16.7) | 35 (83.3) | |
| Community | 6 (22.2) | 21 (77.8) | |
| Healthcare | 15 (41.7) | 21 (58.3) | |
| Duration of hospitalization, d | 25.29 ± 45.88 | 20.34 ± 23.9 | 0.498‡ |
| Prosthetics | 10 (35.7) | 12 (15.6) | 0.025† |
| Considerable foci of infection | 6 (21.4) | 21 (27) | 0.038† |
| Receipt of blood and blood derivatives | 18 (64) | 30 (39) | 0.021 |
| Antibiotic resistance | 0.66† | ||
| MSSA | 18 (64.3) | 53 (68.8) | |
| MRSA | 10 (35.7) | 24 (31.2) | |
| Underlying diseases | |||
| Cardiovascular diseases | 17 (22.4) | 8 (28.6) | 0.511† |
| Diabetes mellitus | 21 (27.3) | 10 (35.7) | 0.402† |
| Hypertension | 35 (45.5) | 17 (60.7) | 0.157† |
| Neurological diseases | 21 (27.3) | 15 (53.6) | 0.012† |
| End-stage renal disease | 14 (18) | 17 (60.7) | 0.001† |
| Immunocompromised | 19 (24.7) | 6 (21) | 0.73† |
| Patient’s outcome | 0.119† | ||
| Death | 34 (45.9) | 6 (27.3) | |
| Presence of SIRS at admissions | 8 (61.5) | 20 (36.4) | 0.097† |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
aValues are expressed as No. (%) or mean ± SD.
b*, The results of independent samples t-test; †, the result of chi-square test; ‡, the result of Mann-Whitney U-test.
| Variable | Discharged | Dead | P-Valueb |
|---|---|---|---|
| Sex | 0.953 | ||
| Male | 29 (53.7) | 20 (37) | |
| Female | 27 (52.9) | 20 (39.2) | |
| Source of infection | 0.654 | ||
| Hospital | 24 (51.7) | 18 (42.9) | |
| Community | 18 (69.2) | 8 (30.8) | |
| Healthcare | 14 (50) | 14 (50) | |
| Antibiotic resistance | 0.614 | ||
| MSSA | 35 (56.5) | 27 (43.5) | |
| MRSA | 21 (61.8) | 13 (38.2) |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
aValues are expressed as No. (%) or mean ± SD.
bThe results of chi-square test.
5. Discussion
The results of the present study on 105 cases showed positive MRSA in 23% of the cases. This rate is within the range reported by a previous meta-analysis of Iranian studies (20.5% - 90%) (12) and similar to that reported on strains with the capability of producing toxic shock syndrome (25.2%) (13). Also, another recent meta-analysis of 23 studies (2007 - 2019) reported MRSA in 22% of children with S. aureus (19), which is close to that reported in the present study; however, the study population differed. There are also reports of higher MRSA in Iranian studies, like the recent study in two geographical regions of Iran (2020), which reported MRSA in 40.8% of isolates in Tabriz and 57.1% of Kerman (14). The difference rates reported can be attributed to the differences in the characteristics of the study populations as well as different diagnostic methods used for the assessment of MRSA. In addition, although the prevalence of MRSA reported in the above-mentioned reports are close to that of the present study, the origin of samples was unclear in these review studies (12, 13, 19), which is of great significance, as the origin of infection is associated with adverse health outcomes and increased economic burden of MRSA (20-22).
The results of the present study showed that 40% were HAI, 25.7% CAI, and 34.3% HCAI. These results are in line with that of large population-based studies, indicating the changing prevalence of the origin of SABSI, while the trend of changes differs among different populations (23, 24). In a nationwide study in the USA, healthcare-associated community-onset infections compromised the greatest proportion (25), while in the present study, HAI and HCAI included the commonest origins. We also found a significant difference in the frequency of MRSA according to the origin of infection; 42.9% of cases with HAI, 33.3% of CAI, and 19.4% of HCAI were MRSA. These results indicate the high rate of MRSA in the study population, especially in cases with HAI and CAI, which can be justified by the suggestion of the different genetic and phenotypic characteristics of the origin of MRSA (16, 26).
The meta-analysis on Iranian children also reported a higher rate of MRSA in nosocomial infections, compared with CAI (38% vs. 17%) (19). These results are consistent with that of the present study; however, the age of the study populations differed. Another meta-analysis of Iranian children also reported a higher pooled prevalence of MRSA in hospitalized children (51%), compared with that in overall patients with a positive S. aureus culture (42%) and healthy children (14%) (27). Additionally, the present study also showed a longer duration of hospitalization based on the origin of the infection (HAI, CAI, and HCAI, respectively), which is consistent with the results of previous studies, emphasizes the significance of MRSA in hospitalized patients (20, 22).
Another important complication of SABSI, addressed in the present study, is infective endocarditis, considered responsible for the great increase in infective endocarditis-related hospitalizations in different nations, like the United States (28) and France (29). The results of the present study showed that 26.6% of the studied patients had infective endocarditis; 2.85% definite, and 23.8% probable. The overall rate of definite infective endocarditis in the present study is lower than in the previous reports. In a retrospective analysis of 465 episodes of S. aureus bacteremia, definite infective endocarditis was found in 8.2% of the population (30). Another study on 244 Danish patients with S. aureus bacteremia reported even a much higher rate (22%) (31). These differences can be due to the difference in the diagnostic methods and criteria, as well as the difference in the frequency of risk factors in the study populations. As demonstrated by the results of the present study, patients who received blood (products), had prosthetic devices, and a considerable focus of infection had a higher rate of infective endocarditis (definite and probable). Moreover, most patients with infective endocarditis had HCAI. These results suggested the risk factors that predispose patients with SABSI to infective endocarditis.
The significance of the origin of infection has also been suggested by Selton-Suty et al. (29) as an important predictor of infective endocarditis in patients with SABSI, which is consistent with the results of the present study. Rasmussen et al. (31) reported the incidence of infective endocarditis at 38%, and Le Moing et al. (32) reported infective endocarditis in 33% of patients with prosthetics, which are close to that reported in the present study (35.7%). In addition to the factors mentioned in the present study, other risk factors have also been suggested for the incidence of infective endocarditis in patients with SABSI, such as intravenous drug abuse (33) and history of embolic events and previous infective endocarditis (34). According to the significance of infective endocarditis in SABSI, some have suggested the use of clinical factors, such as time to a positive culture, in order to estimate the risk of infective endocarditis in patients with SABSI, in order to reduce the risk of mortality by on-time diagnostic and therapeutic strategies (30, 35).
The final outcome of the patients evaluated in the present study was the in-hospital mortality rate, and the results showed that 38.1% of the patients died during their admission. Inagaki and colleagues reported the rate of in-hospital death at 13% in all patients with SABSI in a large population (92,089 patients) (36), which is much lower than that of the present study. They also reported in-hospital mortality in 14.1% of MRSA cases (36), which is again much lower than that of the present study (38.2%). Regression analysis in the study by Inagaki et al. (36) showed that MRSA increased the risk of in-hospital mortality; however, we did not find any statistically significant difference in the mortality rate of MRSA and MSSA groups. This difference could be due to the smaller number of samples in the present study. In addition, the results of the present study could not show any role for age, underlying diseases, and other predictors of death in patients with SABSI, such as the source of infection, suggested by the previous studies (10, 11). This difference could be because of the fact that they have considered the overall mortality rate, which could have different risk factors than in-hospital mortality. There is, unfortunately, little data available about the mortality of patients with SABSI in Iran to be comparable to the results of the present study (37).
The present study had the strength of simultaneous evaluation of multiple demographic, clinical, and paraclinical variables, which enabled studying the association of different variables; especially categorizing patients based on the origin of infection was an important element in the present study, which has been missed in some of the previous studies. However, this study had some limitations, as well. The retrospective evaluation of the variables was one of the limitations of the present study, which disabled studying the correlation between the study variables. Although a wide range of variables was included in this study, there are some confounders that can influence the study outcomes, which have not been included in the analysis, such as injecting drug abuse. The small sample size and enrollment of patients from two hospitals in one city were other limitations of the present study; therefore, generalization of the results of the present study to the whole population should be performed with caution.
5.1. Conclusions
In summary, the results of the present study emphasized the significance of MRSA in patients with SABSI and outlined the origin of infection as a significant factor associated with MRSA. Evaluation of the origin of infection in this study determined this factor as a significant predictor of clinical outcomes, such as duration of hospital stay and MRSA. Furthermore, the results of our study showed the high incidence of infective endocarditis in these patients, which refers to the necessity of paying greater attention to the on-time diagnosis of infective endocarditis in these patients, especially in high-risk patients, such as those with prosthetics. The final outcome of the study population in our study revealed a high in-hospital mortality rate in this population. Further studies with a larger sample size and longer follow-up are required for a better estimation of SABSI in the Iranian population.