1. Background
Chronic kidney disease (CKD) is a worldwide problem influencing public health, with the continuous rising of the number of patients (1). The progressive loss of kidney functions affects the health of the patients and potentially leads to renal replacement therapy (RRT) by dialysis (hemodialysis or peritonea1 dialysis) or kidney transplant. Given the advantages and disadvantages of various treatments available, a specific treatment is ultimately recommended to the patients (2, 3). The infection risk in hemodialysis patients is considerable, explained by impaired immunity and the need for frequent hospitalizations and surgical interventions. Besides, a special type of blood-borne viral infection, i.e., Hepatitis C Virus (HCV) infection, is nearly common in hemodialysis patients (4). Hepatitis C virus, hepatitis B virus (HBV), and human immunodeficiency virus (HIV) can occur due to specific hemodialysis conditions. Hepatitis C virus is an enveloped, single positive-strand RNA virus. It is a member of the Flaviviridae family, Hepacivirus genus (5).
There are seven different genotypes of HCV, divided into several subtypes. Each of these genotypes is prevalent in a certain country, but generally, genotype 1 (Gt 1) is the most prevalent one worldwide (6). Also, several studies in different countries showed that the HCV genotype patterns are different among hemodialysis patients, but in general, subtype 1a appears to be more frequent among hemodialysis patients than in the general population (7). Hemodialysis patients are the highest risk group for the acquisition of HCV infection (8). In Iranian hemodialysis patients, the prevalence of HCV infection is higher than that in the general population (9, 10). The prevalence of HCV infection in hemodialysis patients varies from country to country. Some factors such as blood transfusion and the length of dialysis are associated with such high HCV prevalence rates. Nosocomial routes of transmission such as patient-to-patient transmission and the use of contaminated equipment are also influential (11). Furthermore, patients with HCV infection are susceptible to the infection with HIV or other types of hepatitis (12).
Several studies from 12 provinces of Iran using the recombinant immunoblot assay reported the prevalence of HCV infection to be 7.61% in Iranian hemodialysis patients, which is lower than that in other countries (13). Based on the studies, hemodialysis patients infected with HCV have a high rate of liver diseases (14). Besides, HCV is one of the main causes of acute and chronic hepatitis, liver cirrhosis, hepatocellular carcinoma, and related deaths in the general population and hemodialysis patients (15, 16). Reportedly, hemodialysis patients demonstrate a mild liver disease and mild histological features on liver biopsy (17). Considering the high prevalence of HCV infection in the past and the declining rates currently, further investigations are still suggested.
2. Objectives
This study aimed to determine the HCV infection rate, viral load, and genotype patterns in hemodialysis patients referred to Professor Alborzi Clinical Microbiology Research Center (PACMRC) from 2011 to 2018. Although the prevalence of hepatitis C is decreasing in hemodialysis patients in all societies, a new study is needed to evaluate the prevalence of this virus in Iranian hemodialysis patients.
3. Methods
In this cross-sectional study conducted between 2011 and 2018, 131 hemodialysis patients referred to the PACMRC from all hemodialysis centers of Shiraz, Iran, were evaluated for HCV serostatus, viral load, and genotypes. Hemodialysis patients who were positive for hepatitis B surface antigens were excluded from the study. At first, 5 cc of clot blood sample was obtained by venipuncture and then, the samples were centrifuged, and the separated sera were stored in 1.5 mL vials at -70ºC until further examination. In the first step, we checked the antibodies against HCV infection using the GB anti-HCV V4.0 ELISA kit (Hsinchu science Park-Taiwan) for all patients included in the study.
In the next step, HCV RNAs were extracted from 200 μL serum of each specimen using Invitek kit (Berlin- Germany), based on the described guidelines. In the end, the HCV viral load and genotypes were determined in the patients. To perform HCV quantitative tests and genotyping, commercially available one-step TaqMan real-time PCR HCV quantification and genotype kits (Genome Diagnostics Pvt. Ltd., Hague, Netherland) were used, according to the manufacturer’s instructions using a 7500 Real-Time PCR system (Applied Biosystems, USA). The serostatus of HIV, Hepatitis D Virus (HDV), and HBV was assessed in each hemodialysis patient. Informed consent was obtained from all hemodialysis patients included in this study. The study was approved by the Ethics and Research Committee of CMRC, Shiraz University of Medical Sciences, Shiraz, Iran.
3.1. Statistical Analysis
The chi-squared test was used to assess genotype patterns in hemodialysis patients. The significance level was set at a P-value of < 0.05. Statistical analysis of the data was performed using SPSS for Windows (Version 16.0, 2007, SPSS Inc., Chicago, IL, United States).
4. Results
A total of 131 patients were entered in the study, consisting of 83 (63.4%) men and 48 (36.6%) women, whose samples were collected from 2011 to 2018. The mean age was 46.26 years, and the youngest and the oldest ones were seven and 87-years-old, respectively (Table 1). The HBV, HIV, and HDV serology tests were negative in all patients. The prevalence of HCV-Ab among hemodialysis patients was 37/126 (29.3%). Five patients were missed. In total, 37 hemodialysis patients were HCV-Ab-positive, of whom 21 had active HCV infection, with HCV positivity in both antibody and PCR assays. Five patients were positive for HCV-RNA but not for antibody, while 84 hemodialysis patients were negative for both HCV-RNA and antibody.
| Variables | Patients |
|---|---|
| Age average (y) | 46.26 |
| Age range | 7 - 87 |
| Sex (male/female) | 83/48 |
| Total | 131 |
Demographic Data of Iranian Hemodialysis Patients with Suspected Hepatitis C Virus Infection
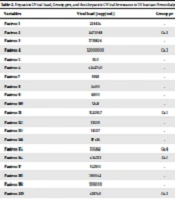
Out of 131 hemodialysis patients included in the study, 71 (54%) had undergone soft organ transplant procedures, of which 69 were kidney transplants, one was a liver-kidney transplant, and one was a liver transplant. On the other hand, 60 (46%) patients had not received any transplant. The HCV treatment rate was lower in HCV-infected hemodialysis patients who underwent organ transplantation (7.2 vs. 20%). Finally, the HCV viral load, genotypes, and the relationship between HCV antibody and viral load were assessed. As shown in Table 2, 30 patients exhibited HCV viremia, and the lowest and the highest viral loads were 950 and 24,086,291 copy/mL, respectively. All the patients were referred four times for HCV tests, among whom 26 patients were positive in the first time and just four patients in the second time. Of the total 30 patients, genotyping was done in 12 patients. The most prevalent HCV genotype was Gt-3, which was detected in six (50%) patients (P ≥ 0.05), followed by Gt-1 in five (41.7%) patients and Gt-4 in one (8.3%) patient. The results suggested that HCV-seronegative patients had lower viral loads than HCV-seropositive counterparts.
| Variables | Viral load (copy/mL) | Genotype | HCV Ab | Transplant |
|---|---|---|---|---|
| Patient 1 | 224436 | - | + | Non-transplant |
| Patient 2 | 2673048 | Gt-3 | + | Kidney |
| Patient 3 | 1739824 | - | + | Non-transplant |
| Patient 4 | 12000000 | Gt-3 | + | - |
| Patient 5 | 950 | - | + | Kidney |
| Patient 6 | 6262760 | - | + | Kidney |
| Patient 7 | 1998 | - | _ | - |
| Patient 8 | 2600 | - | _ | Non-transplant |
| Patient 9 | 4800 | - | + | Kidney |
| Patient 10 | 5168 | - | _ | Kidney |
| Patient 11 | 1522957 | Gt-1 | - | Non-transplant |
| Patient 12 | 13500 | - | + | - |
| Patient 13 | 14107 | - | _ | Kidney |
| Patient 14 | 87614 | - | + | Non-transplant |
| Patient 15 | 110242 | Gt-4 | + | Kidney |
| Patient 16 | 636333 | Gt-1 | - | Non-transplant |
| Patient 17 | 152100 | - | + | Kidney |
| Patient 18 | 190062 | - | + | - |
| Patient 19 | 309000 | - | + | Kidney |
| Patient 20 | 618760 | Gt-3 | _ | - |
| Patient 21 | 700000 | Gt-3 | + | Non-transplant |
| Patient 22 | 1509800 | - | + | - |
| Patient 23 | 3034714 | Gt-1 | + | Non-transplant |
| Patient 24 | 3454450 | - | + | - |
| Patient 25 | 10944150 | Gt-3 | + | Kidney |
| Patient 26 | 12045450 | Gt-3 | + | Non-transplant |
| Patient 27 | 4281515 | Gt-1 | - | - |
| Patient 28 | 5652737 | - | + | Non-transplant |
| Patient 29 | 11570817 | - | + | Kidney |
| Patient 30 | 24086291 | Gt-1 | + | Kidney |
Hepatitis C Viral Load, Genotypes, and Anti-hepatitis C Viral Serostatus in 30 Iranian Hemodialysis Patients
5. Discussion
Despite the currently developed prevention protocols, the risk of HCV transmission has not been eliminated. This requires more careful consideration of health protocols to prevent transmission of HCV to hemodialysis centers by medical staff and patients (18-22). Also, information about the immunological status of patients is necessary, which helps prevent infection transmission. Therefore, the periodic screening of patients is important because most patients are asymptomatic (23). Furthermore, underlying diseases such as chronic renal failure and diabetes are the risk factors of HCV in patients (24).
This study showed a high prevalence of HCV infection in Iranian hemodialysis patients that is higher than that in the general population (33.3 vs. 0.4%) (25). Also, a study performed in the general population of Peshawar, Pakistan, revealed an HCV prevalence of 13.4%, which is less than that in Iranian hemodialysis patients (26). However, similar to Iran, a systematic review and meta-analysis study from Pakistan in 2020 showed a high prevalence of HCV (32.33%) in hemodialysis patients (27). Another study in Al Gharbiyah, Egypt, by the third-generation enzyme immunoassay in 2011 suggested that 42.2% of hemodialysis patients were anti-HCV reactive, which is higher than the rate in Iranian hemodialysis patients (28).
In a study from 10 Middle East countries, the prevalence of HCV infection was found to be 25.3% in hemodialysis patients. The highest reported rates belonged to Egypt and Syria, while Iran and Lebanon had the lowest (29). Furthermore, in a study by Moini et al., the rate of HCV infection was high in HCV-Ab-negative hemodialysis patients. Besides, 7.2% of HCV-Ab-negative patients were positive for HCV core Ag (30). The presence of HCV-RNA in patients with negative serologic results is referred to as occult hepatitis C infection (31). Our study showed that the percentage of Occult HCV Infection (OCI) was 3.9%. Consistent with our study, Naghdi et al. studied the prevalence of OCI among Iranian chronic hemodialysis patients and showed that 3.03% of the patients had positive peripheral blood mononuclear cell HCV-RNA (31).
The present study suggested that the viral load was lower among HCV-seronegative patients than in HCV-seropositive patients, consistent with other studies performed in other countries. For example, Hanuka et al. showed that HCV viremia was very low among anti-HCV-negative hemodialysis patients (32). According to the present study results, the most prevalent HCV genotype was Gt-3 (50%), while Gt-4 was the lowest (8.3%). However, a systematic review and meta-analysis study by Khodabandehloo and Roshani in Iran in 2014 showed subtype 1a was predominant (39%), and genotype 2 with a rate of 3.6% was the lowest (33). Jamalidoust et al. in 2014 showed that the most prevalent genotype was Gt-1 among high-risk groups such as hemodialysis patients (34).
Similar to our study, another study on hemodialysis patients in Tehran in 2006 suggested that subtype 3a was predominant (30.3%) (35). Also, a study performed by Rafiei et al. showed that Gt 3a (51.1%) was the most prevalent genotype among hemodialysis patients (36). A further study on the Iranian general population from March 2010 to March 2012 suggested that Gt 1b (71.1%) was the predominant genotype, and Gt 1a (1.7%) was the lowest (37). Khaja et al. considered HCV genotypes in the general population and chronic renal failure (CRF) patients, and showed that Gt 1b as 43.4% in the general population and Gt 1a as 16.6% among CRF patients were the most prevalent (38). As one of the common reasons for liver transplantation in end-stage cirrhosis is HCV infection, it is important to investigate the effect of HCV infection among the recipients.
As revealed in this study, 79% of HCV patients survived after one year but after five years, the survival rate was the same among those with transplantation with or without HCV infection (39). Also, a study in 2000 suggested that in 75 - 90% of the patients with chronic HCV infection, cirrhosis developed within five years (40). Fortunately, the new treatment regimen against hepatitis C infection and the prevention of more severe infections have led to a drastic infection reduction in this group of patients. The high prevalence of HCV infection in Iranian hemodialysis patients provides reliable evidence that indicates the importance of accurate implementation of intervention programs.
5.1. Conclusions
The introduction of stricter rules for screening blood banks, the widespread use of erythropoiesis-stimulating agents instead of blood transfusion, and increased adherence to infection control practices in dialysis units have led to the reduced prevalence of HCV infection in hemodialysis patients.