1. Background
The prevalence of diphtheria cases in Indonesia has attracted global attention over recent years. The World Health Organization (WHO) reported Indonesia as one of the countries with the highest prevalence of diphtheria cases (1). In 2017, when a diphtheria outbreak occurred, the Indonesian Ministry of Health declared that the total cases of the disease (clinical and laboratory-confirmed) reached 954 persons. East Java Province accounted for the highest prevalence (n = 331; 34.7%). The second to the fifth most prevalent cases were West Java, Banten, Aceh, and Jakarta, respectively, three of which (namely Jakarta, Banten, and West Java) were in the overlapping area surrounding the capital of Indonesia and accounted for 351 cases (36.8%). The government has spared efforts to prevent the disease; however, the cases can still be noticed (2).
The causative agents of diphtheria are the toxigenic strains of Corynebacterium diphtheriae, C. ulcerans, and C. pseudotuberculosis. Corynebacterium diphtheriae is grouped into four subtypes: gravis, mitis, intermedius, and belfanti (3, 4). On the other hand, molecular typing (eg, ribotyping and sequence typing) provides a more detailed description of the relationship between the strains (3, 5). The microbiological aspect of the diphtheria-inducing bacteria contributes to tracing the disease transmission and case management (6). Moreover, the clinical aspects of cases contribute to updating the clinical manifestation, management, and prognosis of the disease, which may not sometimes fit the theory (3, 5). Several previous studies have addressed the microbiological and clinical aspects of diphtheria cases in Indonesia (7, 8). However, the microbiological aspects are generally limited to subtype, toxigenicity, and susceptibility. On the other hand, the clinical aspects generally do not differentiate between clinical diphtheria and diphtheria-confirmed cases. Accordingly, the misdiagnosis of clinical diphtheria is likely to occur.
2. Objective
This study aimed to identify the microbiological and clinical aspects, including molecular typing and case-fatality rates, in diphtheria-confirmed cases from the capital city of Indonesia, Jakarta, and its surrounding areas in 2017.
3. Methods
3.1. Sample and Data Collection
The study was descriptive-analytic in terms of the research design. To reach the aforementioned research objective, the microbiological aspect was obtained from the re-identification of the archive samples in the form of diphtheria-inducing bacteria isolated from 40 diphtheria-confirmed cases reported in Jakarta, Banten, and some regions of West Java Provinces (located in the borderline of Jakarta), including Bogor, Depok, and Bekasi Districts in 2017. The clinical aspects of the diphtheria cases were obtained from the medical records and epidemiological investigation documents. Table 1 presents the diphtheria-confirmed cases’ characteristics.
| Cases Characteristics | No. (%) |
|---|---|
| Age (y) | |
| ≤ 5 | 7 (17.5) |
| 6 - 10 | 11 (27.5) |
| 11 - 15 | 9 (22.5) |
| > 15 | 13 (32.5) |
| Male: Female | 12:28 (30:70) |
| Complete basic vaccination (DTP3) | 5 (12.5) |
| Incomplete basic vaccination | 12 (30) |
| Unvaccinated/uncertain | 23 (57.5) |
| Known: Unknown epidemiology link | 11:29 (27.5:72.5) |
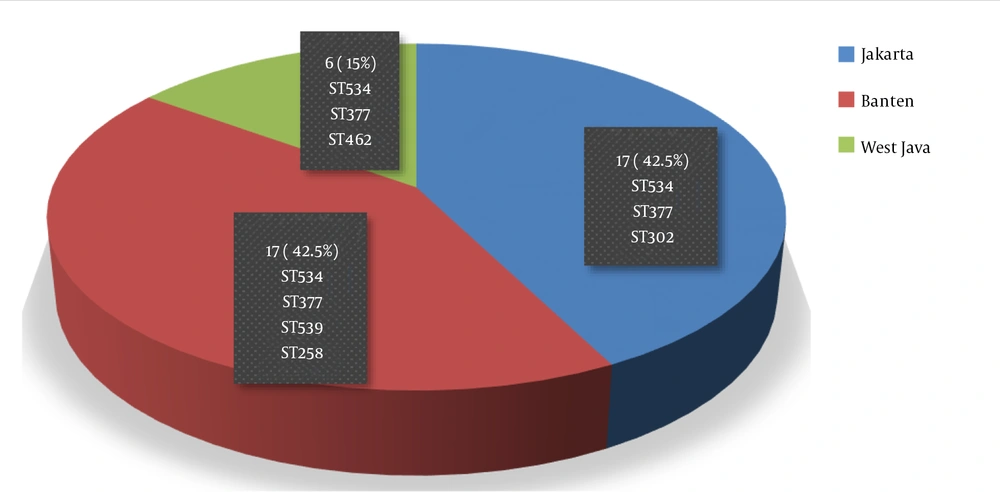
Most of the diphtheria-confirmed cases were < 15-years-old (67.5%), with the age range of 6 - 10 years being the most prevalent one (27.5%) (Table 1). Females were much more than males, with a proportion of 7:3. Most of the cases (70%) had incomplete basic vaccination or unvaccinated history, and about 72% of the cases had unclear historical contact with other cases or sources of disease transmission. The cases from Jakarta and Banten were equally distributed in terms of proportion, and they dominated the other cases (85%) (Figure 1). Furthermore, the data on ethnicity and religious aspects were incomplete, and thus, they were not analyzed in this study.
3.2. Laboratory Examination
The archive samples of diphtheria-inducing bacteria isolates were preserved in TSB + Glycerol medium at the ultra-low temperature. The isolates were revived on a blood agar plate and incubated overnight at 37°C. Then, pure colonies were taken for microscopic identification, as well as biochemistry and toxigenicity tests. Biochemical tests used a commercial Coryne API kit (bioMerieux), while bacterial toxigenicity was determined by PCR and a modified Elek test. The molecular typing of diphtheria-inducing bacteria was conducted using the Multilocus Sequence Typing (MLST) approach. The DNA sequencing was also carried out using the whole genome sequencing (WGS) method, followed by data conversion and analysis using U-gene software. The isolates' Sequence Types (STs) were obtained by online data analysis using the global MLST database (PubMLST), as described in a previous study (9).
3.3. Data Analysis
The collected data were analyzed using SPSS version 16.0 software. The chi-square test was run to determine the correlation between the fatal cases and antitoxin (DAT) administration delay, as well as the correlation between fatal cases and myocarditis. In this study, P ≤ 0.05 was set as the significance level.
4. Results
4.1. Microbiological Aspects
Corynebacterium diphtheria was the only causative agent noticed in the present study, and C. ulcerans and Corynebacterium pseudotuberculosis were not observed (Table 2). The typical diphtheria-inducing bacteria were toxigenic with two subtypes, namely intermedius and mitis. The MLST approach extracted six sequence types from the 28 examined isolates, including ST258, ST302, ST462, ST539, ST377, and ST534. The two last sequence types (namely ST534 and ST377) were dominant in most of the diphtheria cases, where one of the sequence types (ST534) had been reported in previous studies (9). Moreover, ST534 and ST377 were distributed in all provinces based on the geographical area, while the others had a limited distribution (Figure 1).
| Microbiological/Clinical Aspects | No. (%) |
|---|---|
| Microbiological | |
| Corynebacterium diphtheriae | 40 (100) |
| Mitis | 16 (40) |
| Intermedius | 24 (60) |
| Gravis and belfanti | 0 (0) |
| C. ulcerans and C. pseudotuberculosis | 0 (0) |
| Toxigenic | 40 (100) |
| Non-toxigenic/NTTB | 0 (0) |
| Sequence typing a | 28 (70) |
| ST534 | 13 (46.4) |
| ST377 | 10 (35.7) |
| Others | 5 (17.9) |
| Clinical | |
| Fever | 29 (72.5) |
| Sore throat | 31 (77.5) |
| Headache | 11 (27.5) |
| Body temperature > 37°C | 25 (62.5) |
| Cough | 6 (15) |
| Pseudomembrane | 40 (100) |
| Bull neck | 19 (47.5) |
| Airway obstruction | 8 (20) |
| Myocarditis | 3 (7.5) |
| Death | 5 (12.5) |
| Treatment | |
| DAT ≤ 3 days | 5 (12.5) |
| DAT > 3 days | 21 (52.5) |
| Not yet/not given DAT | 14 (35) |
| Penicillin | 9 (22.5) |
| Erythromycin | 3 (7.5) |
| Penicillin + Erythromycin | 3 (7.5) |
| Penicillin/Erythromycin + other Antibiotics | 16 (40) |
| Other antibiotics | 9 (22.5) |
a 28 out of 40 examined isolates.
4.2. Clinical Aspects
Most of the diphtheria-confirmed cases complained of a fever and sore throat in this study (Table 2). On the other hand, all cases had a pseudomembrane, the main clinical sign to diagnose diphtheria in accordance with the WHO guidelines (10), and almost half of the cases demonstrated bull neck as a toxicity indicator. This study also revealed that 20 - 27.5% of the confirmed cases had at least one complication, ie, airway obstruction or myocarditis, with the fatal cases accounting for about 12.5% of the patients. Diphtheria antitoxin (DAT) and antibiotics are introduced as the main therapy for diphtheria cases (3, 11). Sixty-five percent of the cases received DAT therapy; however, most of the patients were administered DAT after the third day from the onset (Table 2). In addition to DAT, the cases were treated with antibiotics such as penicillin and erythromycin (ie, the two first-line antibiotics recommended for diphtheria) to eliminate the causative agent (12). In this study, most of the cases received a combination of penicillin or erythromycin with other antibiotics (40%), and 22.5% of the cases were given only penicillin. Furthermore, although several patients were suffering from airway obstruction, none of the cases received a surgical tracheostomy procedure.
4.3. Statistical Analysis
Statistical analysis showed that the fatal cases were significantly correlated with myocarditis at P = 0.000 (≤ 0.05) (Table 3). However, the fatal cases were not correlated with the delay of DAT administration statistically (P > 0.05).
| Variable | No. | Fatal Cases (%) | P-Value |
|---|---|---|---|
| Myocarditis | 0.00 | ||
| Yes | 3 | 3 (100) | |
| No | 37 | 2 (5.4) | |
| DAT administration | 0.4 | ||
| ≤ 3 days from onset | 5 | 0 (0) | |
| > 3 days from onset or after not receiving DAT (yet) | 35 | 5 (14.3) |
5. Discussion
Some studies have presented a microbiological and clinical overview of diphtheria (7, 13); however, an analysis of laboratory-confirmed cases is rare. Some previous studies have also illustrated diphtheria-confirmed cases with small sample sizes; however, some others have presented a partial overview in separated aspects (12, 14). Herein, we described the microbiological and clinical aspects of diphtheria-confirmed cases. We considered it important because a misdiagnosis risk could be ruled out. The characteristics of diphtheria-confirmed cases in this study, especially in terms of age and vaccination history (Table 1), were similar to those of national diphtheria cases reported by Indonesia’s Ministry of Health (2).
Age proportion could describe vaccination coverage in a country or region. Higher vaccination coverage in a country presents more cases in adolescents aged above 15 years, and lower vaccination coverage may lead to a higher incidence rate in children (15-17). This study indicated that vaccination coverage required to be improved. Although an annual report revealed its high coverage, we predicted the real condition to be different. A previous study showed that the high seroprotective level of antibodies was induced by natural immunity rather than vaccination coverage (18, 19). Furthermore, we detected no definite cause of its high distribution in females. Some previous studies reported similar and different results (12, 20, 21).
Only 12.5% of the cases received complete basic vaccination (DTP3) (Table 1). Vaccination history is typically linked to the disease severity and mortality rates since the diphtheria toxoid vaccine-induced antibody response prevents the toxicity of diphtheria toxin. A study in India revealed that most diphtheria cases were non-vaccination (79%) or incomplete vaccination (18%), with CFR in 48% and tracheostomy in approximately 50% of the cases (22). Nawing et al. also described fatal cases that had not received a vaccination or had received it partially (23). Furthermore, three-quarters of the cases had unidentified contact. This implies that the circulation of bacterial agents and asymptomatic carriers might be high in the environment (6). In this study, toxigenic C. diphtheriae was the only isolated diphtheria-inducing agent (Table 2) generally discovered in developing countries. As known, C. ulcerans is frequently reported in developed countries with high vaccination coverage (6, 24-27) while C. pseudotuberculosis inducing diphtheria in humans has been rare to date. Both C. ulcerans and C. pseudotuberculosis are the causative agents of zoonotic disease (26, 28). This study identified two subtypes, namely intermedius, and mitis, while intermedius domination had been rarely noticed in other countries, except for Brazil and India (20). The non-toxigenic tox gene bearing (NTTB) type, as the potential causative agent of diphtheria, was not also noticed in this study (6).
There were some Sequence Types (ST) discovered, two of which dominated others (Table 2). We found ST377, previously identified in other countries such as India, a notable country with the highest diphtheria cases (17). Secondly, ST534 is the new sequence type that has not been reported from other countries (9). Other types were observed in small proportions. Interestingly, we identified a sequence type (ST302) reported also in Malaysia and the Philippines as neighboring countries. It indicates the transmission of diseases among countries (5). From a clinical perspective, the cases did not always exhibit the two main diphtheria symptoms (namely fever and sore throat) simultaneously (Table 2). The clinicians should be aware of this issue when detecting the likelihood of diphtheria in patients with a fever and no sore throat or vice versa. Phalkey et al. reported coughing as the most clinical manifestation in their study (21); however, this symptom was observed in 15% of the cases in the present study. On the other hand, as an alarming symptom, almost 50% of the cases exhibited bull neck as a toxic sign affecting prognosis (29). Furthermore, although only three cases (7.5%) had myocarditis, awareness is required since it is correlated with fatal cases (7, 30). All cases with myocarditis complications passed away in this study.
Previous studies suggested DAT administration in the first two days after onset (11) since DAT binds to the toxin only before attaching it to cells or tissues. Unfortunately, Indonesia experienced DAT scarcity as such a limited number of patients received DAT in 2017. Accordingly, DAT was mainly administered three days from the onset (Table 2). Among the five fatal cases, three patients were given antitoxin after the third day, and two others were not given DAT and have not received it yet. The lack of an antitoxin was a critical issue. The DAT scarcity occurring in the last few years globally are associated with high fatality rates (20, 31, 32). A recent study proposed using a human monoclonal antibody as an alternative to DAT (33). In this study, penicillin was used more for diphtheria treatment than erythromycin (Table 2). For the hospitalized diphtheria cases, drug delivery injection was often necessary, while erythromycin injection is currently unavailable in Indonesia. Awareness is required because recent studies in Indonesia indicated a decrease in the susceptibility of C. diphtheriae to penicillins (8).
The statistical analysis results support the conclusion indicating that the prevalence rate of myocarditis is always correlated with the fatal cases of diphtheria (Table 3). On the other hand, delays in administering DAT and non-administration of DAT were not associated with the fatal cases statistically although all fatal cases were not given or delayed in receiving DAT. However, it was not possible to distinguish between patients who died before being treated by DAT and those not treated by DAT because of their clinically mild condition. This limitation might also have been caused by the small sample size. Finally, since no case-control research design was adopted, this study could not define the risk factors of the disease. This study could not also represent Indonesia nationally due to the limited research area. These are some limitations to the present study that need to be addressed in future studies.
5.1. Conclusions
The confirmed cases from Jakarta and surrounding areas in 2017 were suffering from diphtheria induced by toxigenic C. diphtheriae with two subtypes (namely, mitis and intermedius) and two main sequence types (namely, ST534 and ST377). It was also concluded that fatal cases were correlated with myocarditis complications.