1. Background
Acinetobacter is a Gram-negative pathogen, whose potentials in inducing various nosocomial infections in immunocompromised patients has attracted much attention worldwide (1). Before 1970, Acinetobacter baumannii was sensitive to most antibiotics, including extended-spectrum beta-lactams (ESBL). However, its capability to acquire resistance genes posed considerable problems in antimicrobial treatments (1, 2). Nowadays, A. baumannii is one of the six most crucial multidrug-resistant (MDR) bacteria in hospitals worldwide (3). Antibiotic resistance is a worldwide threat with an increasing trend and high mortality rates (4), which is rapidly increasing among clinical isolates, especially Acinetobacter spp., Klebsiella spp., Pseudomonas aeruginosa, Proteus spp., Escherichia coli, and Enterobacter spp., because of mobile genetic elements (MGE). Accordingly, isolating resistant bacteria and identifying their resistance mechanisms seem to be crucial. Quick and reliable results are required to apply appropriate therapeutic strategies and prevent the spread of resistance factors in hospitals and the environment (5).
Beta-lactam antibiotics are a large class of antibiotics containing the β-lactam ring in their structure and include penicillins, cephalosporins, monobactams, and carbapenems. Carbapenems are often used as the last line to treat infections caused by MDR Gram-negative bacilli (6). Carbapenems are bactericidal agents acting quickly to kill bacteria and having a more comprehensive range of antimicrobial activities than other antibiotics. These antibiotics (except for Ertapenem) are active against clinically-pathogenic bacteria such as P. aeruginosa, Burkholderia cepacia, and Acinetobacter spp. (7). For years, carbapenems have been an option for treating different infections caused by A. baumannii. Recently, resistance to carbapenems has increased among the clinical and environmental isolates of A. baumannii. (8). Resistance to carbapenems happens through the production of VIM, IMP, NDM, and SIM metallo-beta-lactamases (carbapenems) or non-metallo-carbapenems (9, 10).
The IMP, AmpC, VIM, and TEM beta-lactamase genes are more common in Gram-negative bacteria, while the PER, VEB, and SHV beta-lactamase genes are rarely noticed (10, 11). It is of paramount importance to study these genes since they can be transferred among widely different bacteria from one region and country to another through MGE (10). Accordingly, the regular/periodic investigation of the antibiotic-resistant pattern is necessary to control and reduce emerging new antimicrobial resistance.
2. Objectives
The present study aimed to determine antibiotic-resistant patterns and evaluate the presence of Carbapenem-resistant beta-lactamase genes in the clinical isolates of A. baumannii from patients referred to the Shahid Kamyab Hospital, Mashhad, Iran.
3. Methods
3.1. Sample Collection and Identification
In this study, 286 samples were collected and identified using the GNA macrogen from patients admitted to different wards of Shahid Kamyab Hospital in Mashhad from March 2017 to June 2017. Among the collected samples, 31 samples were confirmed to be A. baumannii.
3.2. Antimicrobial Susceptibility Tests
Antibacterial susceptibility testing was performed using the disk-diffusion (Kirby-Bauer) method according to the Clinical and Laboratory Standards Institute (CLSI, 2014) guidelines (12). Thirteen antimicrobial agents (HiMedia, India) in this study were Imipenem (10 µg), meropenem (10 µg), amikacin (30 µg), ceftriaxone (30µg), ciprofloxacin (5 µg), cefixime (5 µg), polymyxin b (300 units), gentamicin (10 µg), ceftazidime (30 µg), cefotaxime (30 µg), amoxiclav-co-trimoxazole (20/10 µg) and cephalexin (30 µg). Moreover, the isolates were categorized as sensitive, intermediate, and resistant.
3.3. DNA Extraction
The DNA extraction was performed using the boiling method. The extracted DNAs were stored at 4°C. Aliquots of 2 μL of template DNA were used for PCR.
3.4. Polymerase Chain Reaction
The distribution of beta-lactamase genes, namely blaAmpC, blaPER, blaVEB, blaTEM, blaVIM, blaIMP, and blaSHV, were investigated in all A. baumannii isolates by polymerase chain reaction (PCR) using Taq DNA polymerase master mix (Amplicon, Denmark). The primer sequences (Microgen, South Korea) presented in Table 1 were adopted from previous studies. The PCR conditions were as follows: initial denaturation at 95°C for 5 minutes, 30 cycles with denaturation at 94°C for 1 minute, 30-second annealing at 54.5°C for blaSHV and blaVEB, at 50°C for blaAmpC and blaPER, at 56°C, 54°C, and 51°C for blaIMP, blaTEM and blaVIM, respectively, extension at 72°C for 1 minute, followed by final extension at 72°C for 10 minutes (Bio-rad, Germany).
| Gene and | Primers' Sequences | TM (°C) | Length (bp) | Reference |
|---|---|---|---|---|
| blaAmpC | 663 | (13) | ||
| Forward | 5'- ACTTACTTCAACTCGCGACG -3' | 50 | ||
| Reverse | 5'- TAAACACCACATATGTTCCG -3' | 50 | ||
| blaVIM | 390 | (14) | ||
| Forward | 5'- GATGGTGTTTGGTCGCATA -3' | 51 | ||
| Reverse | 5'- CGAATGCGCAGCACCAG -3' | 51 | ||
| blaIMP | 1150 | (15) | ||
| Forward | 5'- CATGGTTTGGTGGTTCTTGT -3' | 56 | ||
| Reverse | 5'- ATAATTTGGCGGACTTTGGC -3' | 56 | ||
| blaTEM | 535 | (16) | ||
| Forward | 5'- AGGAAGAGTATGATTCAACA -3' | 54 | ||
| Reverse | 5'- CTCGTCGTTTGGTATGGC -3' | 54 | ||
| blaPER | 520 | (14) | ||
| Forward | 5'- GCTCCGATAATGAAAGCGT -3' | 50 | ||
| Reverse | 5'- TTCGGCTTGACTCGGCTGA -3' | 50 | ||
| blaVEB | 648 | (14) | ||
| Forward | 5'- CATTTCCCGATGCAAAGCGT -3' | 54.5 | ||
| Reverse | 5'- CGAAGTTTCTTTGGACTCTG -3' | 54.5 | ||
| blaSHV | 713 | (17) | ||
| Forward | 5'- AGCCGCTTGAGCAAATTAAAC- 3' | 54.5 | ||
| Reverse | 5'- ATCCCGCAGATAAATCACCAC- 3' | 54.5 |
Primers’ Characteristics
3.5. Gel Electrophoresis
Finally, 5 μL of PCR product and a 100 bp DNA ladder was electrophoresed using 1% agarose gel to confirm the PCR amplification.
4. Results
4.1. Identification of Bacterial Isolates
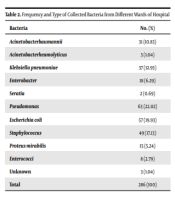
Out of 286 isolates (Table 2), 31 isolates (70.96% from males and 29.03% from females) were A. baumannii. The A. baumannii isolates were isolated from the patients’ wound (n = 10), lungs (n = 10), throats (n = 4), blood (n = 3), bronchi (n = 2), catheters (n = 1), and cerebrospinal fluid (n = 1). Table 3 presents the sources of the isolates, according to which the highest infection source is for intensive care units (ICUs) (77.41%).
| Bacteria | No. (%) |
|---|---|
| Acinetobacter baumannii | 31 (10.83) |
| A. heamolyticus | 3 (1.04) |
| Klebsiella pneumoniae | 37 (12.93) |
| Enterobacter | 18 (6.29) |
| Seratia | 2 (0.69) |
| Pseudomonas | 63 (22.02) |
| Escherichia coli | 57 (19.93) |
| Staphylococcus | 49 (17.13) |
| Proteus mirabilis | 15 (5.24) |
| Enterococci | 8 (2.79) |
| Unknown | 3 (1.04) |
| Total | 286 (100) |
Frequency and Type of Collected Bacteria from Different Wards of Hospital
| Wards | ICU | Women | Neurology | Orthopedics |
|---|---|---|---|---|
| Isolates genes | 24 (77.42) | 3 (9.68) | 2 (6.45) | 2 (6.45) |
| AmpC | 22 (91.66) | 3 (100) | 2 (100) | 2 (100) |
| VIM | 17 (70.83) | 3 (100) | 2 (100) | 2 (100) |
| TEM | 13 (54.16) | 2 (66.66) | 0 (0) | 1 (50) |
| PER | 8 (33.33) | 2 (66.66) | 2 (100) | 1 (50) |
| SHV | 11 (45.83) | 1 (33.33) | 2 (100) | 1 (100) |
| VEB | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IMP | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Frequency and Percentage of Genes and Acinetobacter baumannii Isolates from Different Wards of the Hospital a
4.2. Antibiotic Susceptibility Tests
Table 4 shows the antibiotic resistance patterns in the A. baumannii isolates. All isolates were MDR and revealed resistance to most of the tested antibiotics. The highest resistance level (100%) was observed against imipenem, meropenem, ceftazidime, ceftriaxone, cefixime, cefotaxime, and cephalexin. On the other hand, the lowest resistance level was observed against polymyxin B (19.35%). Notably, 92.3% of the isolates were resistant to at least six classes (out of seven) of the antibiotics (MDR).
| Antibiotic Class | Resistant | Intermediate | Sensitive |
|---|---|---|---|
| Carbapenems | |||
| Imipenem (10 µg) | 31 (100) | 0 (0) | 0 (0) |
| Meropenem (10 µg) | 31 (100) | 0 (0) | 0 (0) |
| Cephalosporins | |||
| Ceftazidime (30 µg) | 31 (100) | 0 (0) | 0 (0) |
| Ceftriaxone (30 µg) | 31 (100) | 0 (0) | 0 (0) |
| Cefixime (5 µg) | 31 (100) | 0 (0) | 0 (0) |
| Cefotaxim (30 µg) | 31 (100) | 0 (0) | 0 (0) |
| Cephalexin (30 µg) | 31 (100) | 0 (0) | 0 (0) |
| Quinolones | |||
| Ciprofloxacin (5 µg) | 29 (93.54) | 0 (0) | 2 (6.45) |
| Polymixin | |||
| Polymyxin B (300 unit) | 6 (19.35) | 3 (11.50) | 22 (70.96) |
| Aminoglycosides | |||
| Gentamicin (10 µg) | 25 (80.64) | 0 (0) | 6 (19.35) |
| Amikacin (30 µg) | 22 (70.96) | 1 (3.8) | 8 (25.80) |
| Penicillin | |||
| Amoxyclave (20/10 µg) | 30 (96.77) | 0 (0) | 1 (3.22) |
| Sulfonamides | |||
| Co-tri-moxazol (1.25/23.75 µg) | 24 (77.41) | 2 (7.70) | 5 (16.12) |
Resistance and Sensitivity Frequency and Percentage of Acinetobacter baumannii Isolates to Different Antibiotics a
4.3. Determination of Beta-Lactamase Genes
In this study, blaAmpC, blaVIM, blaTEM, blaSHV, and blaPER were observed in 29 (93.54%), 24 (77%), 16 (51.61%), 15 (48.38%), and 13 (41.93%) A. baumannii isolates, respectively. However, blaVEB and blaIMP genes were found in none of the isolates (0%). Fourteen genotypic patterns were observed among the A. baumannii isolates (Table 5). According to the results, all isolates harbored at least one gene, indicating that the studied beta-lactamase genes were 100% involved in antibiotic resistance. The distribution rates of beta-lactamase genes among the concerned isolates were as follows: One gene in two isolates, two genes in six isolates, three genes in 11 isolates, four genes in 10 isolates, and five genes in two isolates. However, none of the isolates contained six or seven genes together. The highest frequency (n = 26) belonged to the AmpC and VIM genes, and the lowest frequency (n = 13) belonged to the PER gene. Tables 3 and 6 show the frequency of the genotypic pattern and the frequency distribution of genes in the A. baumannii isolates by the type of clinical samples and hospital wards, respectively. Accordingly, the AmpC and VIM were the most frequent genes in the wound and lung samples from the ICU wards. Finally, the results also indicated that group A and C beta-lactamases were with the highest frequency in the isolates.
| Genotype | Frequency Percentage | |
|---|---|---|
| 1 | TEM | 1 (3.22) |
| 2 | AmpC | 1 (3.22) |
| 3 | TEM, AmpC | 3 (9.67) |
| 4 | SHV, VIM | 1 (3.22) |
| 5 | AmpC, VIM | 2 (6.45) |
| 6 | SHV, AmpC, VIM | 3 (9.67) |
| 7 | TEM, AmpC, VIM | 3 (9.67) |
| 8 | PER, AmpC, VIM | 3 (9.67) |
| 9 | PER, TEM, AmpC | 1 (3.22) |
| 10 | SHV, TEM, AmpC | 1 (3.22) |
| 11 | PER, TEM, AmpC, VIM | 2 (6.45) |
| 12 | PER, SHV, AmpC, VIM | 5 (16.12) |
| 13 | SHV, TEM, AmpC, VIM | 3 (9.67) |
| 14 | PER, SHV, TEM, AmpC, VIM | 2 (6.45) |
Prevalence of Different Genotypic Patterns of Beta-Lactamase Genes in Acinetobacter baumannii Isolates
| Gene | Bronchi | Throat | Lung | Blood | Wound | Catheters | CSF |
|---|---|---|---|---|---|---|---|
| AmpC | 2 (100) | 4 (100) | 9 (90) | 3 (100) | 9 (90) | 1 (100) | 1 (100) |
| VIM | 2 (100) | 4 (100) | 7 (70) | 2 (66) | 8 (80) | 1 (100) | 0 (0) |
| TEM | 2 (100) | 2 (50) | 4 (40) | 3 (100) | 4 (40) | 0 (0) | 1 (100) |
| SHV | 0 (0) | 1 (25) | 6 (60) | 1 (33.3) | 6 (60) | 1 (100) | 0 (0) |
| PER | 0 (0) | 1 (25) | 4 (40) | 1 (33.3) | 5 (50) | 1 (100) | 1 (100) |
| VEB | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IMP | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Frequency Distribution of Genes in Acinetobacter baumannii Isolates by Type of Clinical Samples a
5. Discussion
This study aimed to determine antibiotic resistance patterns and investigate the presence of beta-lactamase genes in the clinical isolates of A. baumannii. According to the findings, the most significant proportion of the A. baumannii isolates was from males and the ICU ward of the hospital. This finding is in agreement with the findings of other researchers, including Sharif et al. and Shoja et al. in Iran, Chang et al. and Liu and Liu in China, and Aksoy et al. in Turkey (13, 18-21). The A. baumannii isolates were isolated the most from the wound and lung samples and the least from cerebrospinal fluid and catheters, which is consistent with Aghamiri et al.’s study in Tehran, Iran (22).
Resistance to carbapenems (imipenem and meropenem) and cephalosporins (ceftazidime, ceftriaxone, cefixime, cefotaxime and cephalexin) was observed in all A. baumannii isolates. The imipenem-resistance isolates of A. baumannii were reported to be 26.7, 54, and 100% in studies by Khosroshahi and Sharifi, Peymani et al. and Ibrahimagić et al., respectively (23-25). Resistance to imipenem increased over time from 26.7% in 2007 to 100% in 2017. In the studies conducted in different regions of the world by Peymani et al., Sharif et al., Zarifi et al., Ibrahimagić et al., Al-Hassan and Al-Madboly, and Khuntayaporn et al., 56, 82, 98.6, 30.8, 81, and 98% of A. baumannii isolates exhibited resistance to Meropenem, respectively (18, 24-28). Similarly, resistance to meropenem increased over time from 26.7% in 2011 to 100% in 2021. The comparison of the results indicates that antibiotic resistance is growing over time (29), which could be due to the inappropriate use and the overuse of antibiotics in previous years as well as the increasing prevalence of beta-lactamase genes. Accordingly, antimicrobial therapies have become less effective.
High resistance to carbapenems could be alarming as these antibiotics are often used as the last resort to treat life-threatening infections in humans. In the present study, resistance to Imipenem and meropenem (100%) was considerably higher than values reported in previous studies in different parts of Iran and lower than the results recently reported by Khuntayaporn et al. in Thailand (28). Resistance to polymyxin B was reported to be 0, 10.9, 11, and 16% in A. baumannii isolates by Shoja et al., Abbasi Shaye et al., Saranathan et al., and Ahdi Khosroshahi et al. respectively (19, 30-32). Furthermore, the lowest resistance in the present study was observed against polymyxin B (19.35%).
The PCR results of the present study showed that all isolates contained at least one beta-lactamase gene. The AmpC gene, belonging to the class C beta-lactamases with 93.54%, was the most commonly observed beta-lactamase gene in this study. This finding is in line with those studies reporting the high prevalence of this gene among the clinical isolates of A. baumannii in different regions (13, 33-35). The VIM and the IMP genes are associated with the class B beta-lactamases; however, IMP is not frequently found in A. baumannii isolates regarding the prevalence of beta-lactamase genes. In the present study, the IMP gene was found in none of the isolates, which is in accordance with several studies conducted in Iran and other countries (13, 14, 16, 21, 24, 27, 33, 36, 37).
Previous studies have revealed the low prevalence of VIM; however, it was frequently observed in the A. baumannii isolates in the present study (77%), indicating its increasing prevalence over time. The VIM gene was found in none of the A. baumannii isolates studied by Shoja et al. in Ahvaz, Iran, from 2010 to 2013 (19, 36, 37). However, Farajzadeh Sheikh et al. reported the presence of VIM in 31.4% of A. baumannii isolates in Ahvaz, Iran (38). Moreover, the prevalence of VIM in A. baumannii was reported to be 17.44, 31.4, 36%, 40, and 86% in different countries (22, 38-41). Class A beta-lactamase genes, including SHV, TEM, PER, and VEB, showed different prevalence rates in the A. baumannii isolates (48.38, 51.61, 41.93, and 0%, respectively). Accordingly, VEB was noticed in none of the A. baumannii isolates, which is in accordance with many other studies (14, 25, 34, 42).
Previous studies have reported an increase in the prevalence of SHV in A. baumannii, indicating that it was previously more common in Enterobacteriaceae (43). The prevalence rates of TEM and PER in the present study were 51.61 and 41.93, respectively. This finding is not in line with the findings reported by Zarifi et al. in Mashhad, Iran as they reported the prevalence of TEM and PER in A. baumannii samples to be 27.1 and 7.1%, respectively, showing that an increase in the prevalence of TEM and PER in the clinical isolates of A. baumannii (26). Abdar et al. also reported an increase in the prevalence of TEM (42%) in A. baumannii strains isolated from nosocomial infections in Tehran, Iran (42). The findings indicated that most isolates were resistant to most of the concerned antimicrobials, and that about 92.3% of the isolates were resistant to more than six classes of antibiotics, classified in the MDR group. Moreover, all isolates showed resistance at least to one of the studied antibiotics. The presence of at least one beta-lactamases gene in all isolates confirms the high prevalence of resistance to these antibiotics.
5.1. Conclusions
In conclusion, the findings showed an increase in resistance to some antibiotics, including carbapenems and cephalosporins, reflecting that the high prevalence of beta-lactamase genes among A. baumannii isolates is probably due to the excessive and improper use of antimicrobial agents by patients. The findings also indicated that the highest prevalence rates in the concerned isolates were related to groups A and C beta-lactamases. The high resistance of the isolates to antibiotics indicates the necessity of detecting resistant strains rapidly and timely to select appropriate treatment options and prevent the spread of resistance. Moreover, it is recommended to treat nosocomial infections according to the regional patterns of sensitivity and resistance to prevent the spread of drug-resistant strains.