1. Background
Coronaviruses (CoVs) are enveloped with a positive single-stranded RNA, β-coronavirus belonging to the family Coronaviridae (1, 2). The first human coronaviruses (HCoVs) were detected in 1966; since then, they have been mainly associated with common cold or mild upper respiratory tract infections (2). In the past two decades, the severe acute respiratory syndrome coronavirus (SARS-CoV) outbreak in 2003 and Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in 2012 caused local outbreaks and global pandemics with mortality rates of 10 and 40%, respectively (3).
In December 2019, SARS-CoV-2, the third most pathogenic novel HCoV, was emerged for the first time in Wuhan, China (1, 4, 5). Coronavirus disease 2019 (COVID-19) caused by the SARS-CoV-2 rapidly spread across China and many other countries, and it was declared to be a global pandemic by the World Health Organization (WHO) (6).
The clinical manifestations of COVID-19 are characterized by a variable disease phenotype, of which most patients experience only fever, cough, myalgia, fatigue, or dyspnea. About 15% of patients progress to severe disease, and acute respiratory distress syndrome (ARDS) or multiple organ failure may occur (7-9). SARS‐CoV‐2 infection may trigger a cytokine storm, which is characterized by the excessive production of several proinflammatory cytokines/chemokines and high-level activation of CD4+/CD8+ T cells (10). Several studies suggested that the cytokine storm directly correlated with ARDS, multiple organ failure, disease severity, and death in patients with COVID-19 (1, 9). The unfavorable prognosis of severe COVID-19 is associated with increases in several inflammatory cytokines and chemokines (9).
2. Objectives
We aimed to compare the serum levels of cytokines and chemokines between mild and severe COVID-19 patients.
3. Methods
3.1. Study Population
In this study, a total of 78 patients admitted to the Hospital of Kafkas University, Kars, Eastern Turkey, and Çapa Hospital of Istanbul University, Western Turkey, were included. All subjects tested positive for SARS-CoV-2 in nasopharyngeal specimens by real-time reverse transcription polymerase chain reaction (RT-PCR). The patients were classified into two severe (n = 46) and mild (n = 32) groups according to disease severity definitions by the WHO (10). Demographic, clinical, laboratory, and radiological findings along with management and outcome data of patients were obtained from medical records.
3.2. Samples
After the diagnosis of COVID-19 using RT-PCR, blood samples were collected from all patients. The samples were centrifuged at 1000 xg for 15 minutes at 4°C followed by transfer of plasma to polypropylene tubes. Plasma samples were centrifuged again at 4000 xg for 15 minutes at 4°C to completely remove platelets and precipitates. Plasma samples were diluted fourfold (1: 4), aliquoted, and stored at -80°C until analysis.
3.3. Quantification of Cytokines, Chemokines, and Growth Factors
The serum levels of cytokines, chemokines, and growth factors were determined using the Bio-Plex ProTM Human Cytokine Screening Panel (Bio-Rad, Hercules, CA, USA), which included the following 17 markers; G-CSF, granulocyte-macrophage (GM)- CSF, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, IL-17, MCP-1, MIP-1β, and TNF-α. Serum samples, microbeads, standard curves, and reagents were prepared, and multiplex immunoassays were performed according to the manufacturer’s protocol. Measurements were done using the BioPlex200 Multiplex System platform. The concentrations of cytokine and chemokines were calculated by reference to the standard curve.
3.4. Statistical Analysis
We described the continuous variables as mean ± standard deviation (SD) and the categorical variables as frequency rates and percentages. The normality assumption was tested by Shapiro-Wilk and Kolmogorov-Smirnov tests. The independent group t tests and Mann-Whitney U test were used to evaluate comparison of independent variables that were normally and non-normally distributed, respectively. Categorical variables were compared using the χ2 test. Two-sided P-values less than 0.05 were considered statistically significant. All statistical analyses were performed using R version 3.6.0 program.
4. Results
Out of a total of 78 COVID-19 patients (54 males and 24 females) with confirmed COVID-19 infection, 46 (59%) patients were categorized as severe cases. The mean age was 43.1 ± 13.3 (range: 18 - 65) and 58.2 ± 15 (range: 35 - 92) years in mild and severe groups, respectively. Compared with mildly symptomatic patients, severe patients were significantly older and with a male predominance. The most common symptoms were myalgia or fatigue (67.9%) and cough (46.2%), followed by dyspnea (35.9%) and fever (34.6%). Comorbidities were present in more than half of the patients, with hypertension (33.3%) being the most common, followed by cardiovascular disease (14.1%) and diabetes (14.1%). Severe patients had significantly higher incidence of comorbid diseases. Also, eight patients (17.4%) were admitted to intensive care unit (ICU), of whom six case (75 %) had multiple comorbidities (Table 1).
| Variables | All (n = 78) | Severe (n = 46) | Mild (n = 32) | P-Value |
|---|---|---|---|---|
| Age (y) | 51.7 ± 16.1 (18 - 92) | 58 ± 15 (35 - 92) | 43.1 ± 13.3 (18 - 65) | < 0.001 |
| Sex | ||||
| Males | 54 (69.2) | 36 (78.3) | 18 (56.2) | |
| Females | 24 (30.8) | 10 (21.7) | 14 (43.8) | |
| Comorbid conditions | ||||
| Diabetes | 11 (14.1) | 10 (21.7) | 1 (3.1) | 0.021 |
| Hypertension | 26 (33.3) | 24 (52.2) | 2 (6.2) | < 0.001 |
| Cardiovascular disease | 11 (14.1) | 11 (23.9) | - | 0.002 |
| COPD | 7 (9) | 7 (15.2) | - | 0.037 |
| Malignancy | 1 (1.3) | 1 (2.2) | - | 1 |
| Chronic liver disease | 1 (1.3) | 1 (2.2) | - | 1 |
| Signs and Symptoms | ||||
| Fever | 27 (34.6) | 27 (58.7) | - | < 0.001 |
| Cough | 36 (46.2) | 31 (39.7) | 5 (15.6) | < 0.001 |
| Myalgia or fatigue | 53 (67.9) | 36 (78.3) | 17 (53.1) | 0.027 |
| Headache | 14 (17.9) | 3 (6.5) | 11 (34.4) | 0.003 |
| Diarrhea | 4 (5.1) | 3 (6.5) | 1 (3.1) | 0.64 |
| Dyspnea | 28 (35.9) | 27 (58.7) | 1 (3.1) | < 0.001 |
| Nausea and vomiting | 6 (7.7) | 6 (13) | - | 0.038 |
| Sore throat | 12 (15.4) | 4 (8.7) | 8 (25) | 0.062 |
Abbreviation: COPD, chronic obstructive pulmonary disease.
a Values are expressed as No. (%) or mean ± SD (range).
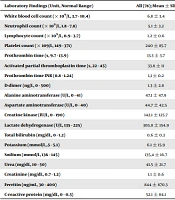
We observed significant differences in hematological and biochemical laboratory findings between the two groups. While neutrophil and white blood cell (WBC) counts were significantly higher in severe patients, lymphocyte and platelet counts were lower in them. Compared with mild patients, severe patients had significantly higher levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), D-dimer, ferritin, C-reactive protein (CRP), and urea (Table 2). On admission, abnormalities on chest computed tomography (CT) were seen in all severe patients. Moreover, 42 (91.3%) patients had bilateral multilobar peripherally distributed ground glass opacities and consolidation areas, of whom 19 (41.3%) had more than 50% of infiltration of the lung parenchyma and four (8.7%) patients had few subpleural small ground glass opacities in one or two lobes.
| Laboratory Findings (Unit, Normal Range) | Mean ± SD | P-Value | ||
|---|---|---|---|---|
| All (78) | Severe (46) | Mild (32) | ||
| White blood cell count (× 109/L, 3.7 - 10.4) | 6.8 ± 3.4 | 7.8 ± 3.7 | 5.4 ± 2.2 | 0.002 |
| Neutrophil count (× 109/L, 1.8 - 7.8) | 5.1 ± 3.2 | 6.1 ± 3.7 | 3.7 ± 1.9 | 0.001 |
| Lymphocyte count (× 109/L, 0.9 - 3.7) | 1.2 ± 0.6 | 1 ± 0.6 | 1.5 ± 0.6 | < 0.001 |
| Platelet count (× 109/L, 149 - 371) | 240 ± 85.7 | 216.2 ± 52.6 | 257.4 ± 100.6 | 0.045 |
| Prothrombin time (s, 9.7 - 13.9) | 13.3 ± 3.7 | 13.1 ± 3.9 | 13.8 ± 2.9 | 0.549 |
| Activated partial thromboplastin time (s, 22 - 45) | 33.8 ± 11 | 33.4 ± 9.5 | 35.1 ± 14.6 | 0.616 |
| Prothrombin time INR (0.8 - 1.24) | 1,1 ± 0.2 | 1 ± 0.1 | 1.2 ± 0.2 | 0.002 |
| D-dimer (ng/L, 0 - 500) | 1.3 ± 2.8 | 1.8 ± 3.4 | 0.5 ± 0.7 | 0.042 |
| Alanine aminotransferase (U/L, 0 - 41) | 47.1 ± 47.9 | 57.5 ± 55.3 | 32.4 ± 29.9 | 0.03 |
| Aspartate aminotransferase (U/L, 0 - 40) | 44.7 ± 42.5 | 57.3 ± 50.9 | 26.6 ± 11.5 | 0.002 |
| Creatine kinase (IU/L, 0 - 190) | 142.1 ± 125.7 | 154.7 ± 137.3 | 108.5 ± 82.1 | 0.228 |
| Lactate dehydrogenase (U/L, 135 - 225) | 305.8 ± 154.9 | 373.3 ± 165.3 | 203.3 ± 40.6 | < 0.001 |
| Total bilirubin (mg/dL, 0 - 1.2) | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.5 ± 0.2 | 0.3 |
| Potassium (mmol/L,.5 - 5.1) | 6.1 ± 15.9 | 7.5 ± 20.8 | 4.1 ± 0.4 | 0.384 |
| Sodium (mmol/L, 136 - 145) | 135,4 ± 16.7 | 132.6 ± 20.9 | 139.7 ± 3.1 | 0.089 |
| Urea (mg/dL, 10 - 50) | 41.5 ± 21.7 | 46.7 ± 24.7 | 33.3 ± 12.6 | 0.013 |
| Creatinine (mg/dL, 0.7 - 1.2) | 1.1 ± 0.6 | 1.1 ± 0.7 | 1 ± 0.3 | 0.323 |
| Ferritin (ng/mL, 30 - 400) | 844 ± 870.3 | 1303.3 ± 860.4 | 171.4 ± 147.3 | < 0.001 |
| C-reactive protein (mg/dL, 0 - 0.5) | 52.1 ± 84.1 | 139.2 ± 84.1 | 1 ± 2.6 | < 0.001 |
All patients received favipiravir, as it is the only approved antiviral for COVID-19 patients in Turkey. Favipiravir was used for five days in mild patients and up to 10 days in severe patients who received supplemental oxygen support additionally. The nasal cannula (38, 82.6%) and non-invasive ventilation or high-flow nasal cannula (15, 32.6%) were the most common procedures applied to severe patients. Invasive mechanical ventilation was required in three (6.5%) patients, of whom two (66.6%) died. In severe patients needing oxygen therapy support due to respiratory distress, 6 mg/day dexamethasone or 0.5 - 1 mg/kg prednisolone was used for up to 10 days. In patients whose oxygen demand increased within 24 hours or whose acute phase response increased despite the treatment, a higher dose of corticosteroids was used after considering the risk factors of the patient. High-dose corticosteroids therapy was used on a case-by-case basis and in some cases for up to three days. After high-dose steroid administration, treatment with 6 mg/day dexamethasone was continued. Out of 46 severe patients, 35 (78.3%) received systematic corticosteroids, 36 (78.3%) antibiotic treatment, and 2 (4.3%) intravenous immunoglobulin therapy.
| Immune Mediator | Severe (n = 46) | Mild (n = 32) | P-Value a | ||
|---|---|---|---|---|---|
| Mean ± SD | [Q1:Q3] Median | Mean ± SD | [Q1:Q3] Median | ||
| IL-6, pg/mL | 60.3 ± 95.3 | [19.50:91.98] 29.6 | 4.3 ± 2.4 | [3.08:5.79] 3.46 | < 0.001 |
| TNF-α, pg/mL | 48 ± 53.9 | [19.28:66.00] 35.94 | 30.7 ± 11.6 | [29.34:45.85] 35.98 | 0.644 |
| IFN-γ, pg/mL | 14.3 ± 26.3 | [3.71:11.49] 5.82 | 6.5 ± 7.1 | [3.01:5.47] 3.01 | 0.271 |
| IL-13, pg/mL | 1.6 ± 0.7 | [1.14:1.63] 1.63 | 1.3 ± 0.3 | [1.14:1.14] 1.14 | 0.119 |
| MCP-1, pg/mL | 159.5 ± 262.8 | [91.92:286.97] 107.35 | 86,4 ± 51.7 | [65.32:126.36] 99.03 | 0.533 |
| IL-8, pg/mL | 59 ± 154.9 | [15.96:51.06] 35.64 | 12 ± 5 | [13.13:17.38] 15.48 | < 0.001 |
| MIP-1β, pg/mL | 54.1 ± 89.2 | [29,51:75,93] 53 | 38.6 ± 15.6 | [41,07:53,33] 43.96 | 0.854 |
a Statistical analysis was performed using median values.
The serum levels of 17 markers were evaluated; however, only TNF-α, IFN-γ, IL-6, IL-8, IL-13, MIP-1β, and MCP-1 levels could be analyzed between severe and mild patients (Table 3). The levels of G-CSF, GM-CSF, IL-2, IL-4, IL-5, IL-7, IL-10, IL-12 (p70), IL-17, and IL-1β were not obtained due to low levels that could not be interpreted using standard curves. Therefore, these biomarkers were excluded from the final analysis. The median levels of IL-8 and IL-6 were significantly higher in severe patients than mild ones (P < 0.001 and P < 0.001, respectively). The median levels of IFN-γ, IL-13, MIP-1β, and MCP-1 were higher in severe patients, although the difference was not statistically significant (P = 0.271, P = 0.119, P = 0.854, and P = 0.533, respectively). In addition, there was no statistically significant difference between the two groups for TNF-α levels (P = 0.644).
5. Discussion
COVID-19 pandemic remains a global health problem with high rates of morbidity and substantial mortality. COVID-19 infection may be accompanied by an uncontrolled inflammatory response that triggers the overproduction of proinflammatory cytokines known as “cytokine storm” (6, 11). The increase in circulating proinflammatory cytokines, including TNF-α, IFN-γ, IL-6, IL-8, and IL-2 results in cytokine storm, which is thought to be associated with disease severity (12, 13). Therefore, recent studies have focused on determining the association between inflammatory responses and severity of COVID-19.
Our findings showed that the main clinical symptoms of severe COVID-19 patients were myalgia, fatigue, cough, dyspnea, and fever. We demonstrated that severe patients were significantly older than mild patients, and they had comorbidities such as hypertension, cardiovascular disease, and diabetes. Severe patients were characterized by significant laboratory abnormalities such as increased WBC and neutrophil counts and decreased lymphocyte and platelet counts. The levels of ALT, AST, LDH, urea, ferritin, D-dimer, and CRP were higher in severe patients. Also, we found that IL-6 and IL-8 significantly increased in severe patients. Accordingly, older age, presence of comorbid conditions, hematological and biochemical laboratory abnormalities, and higher levels of inflammatory cytokines were closely related to disease progression of COVID-19, which is consistent with the results of some previous reports (14).
To date, it has not been explained why some infected patients are asymptomatic, while others have a range of mild, moderate, severe, and critical symptoms. Previous studies suggested that an uncontrolled and overproduction of proinflammatory cytokines/chemokines by immune system might play a major role in severity of MERS-CoV, SARS-CoV, and SARS-CoV-2 infections. The elevated levels of proinflammatory cytokines/chemokines, such as IL-6, IL-8, IFN-γ, and TNF-α were found to be associated with disease severity or mortality in COVID-19 patients, which is similar to SARS-CoV and MERS-CoV infections (15-20). Previous studies have shown that marked elevation of proinflammatory cytokines, such as IL-1, TNF-α, IL-6, TGF-β, and IL-10 were correlated with pulmonary inflammation and severe lung impairment in MERS-CoV patients (20). SARS-CoV infection was also reported to induce ARDS associated with highly expressed proinflammatory cytokines, such as IL-1β, IL-6, IL-8, IL-12, INF-γ, and TNF-α (21-23).
In our study, the serum levels of IL-6, IL-8, IL-13, IFN-γ, MIP-1β, and MCP-1 increased in the severe group compared to the mild group; however, statistically significant differences were observed only in IL-6 and IL-8 levels. Gong et al. demonstrated that higher levels of IL-2R, IL-6, IL-8, IL-10, and TNF-α were associated with COVID-19 progression (24). Karki et al. suggested that serum levels of IL-6 and TNF-α were significant predictors of disease severity and death in COVID-19 infection (25). In addition, a meta-analysis by Akbari et al., including 7,865 COVID-19 patients, showed significant increases in IL-2, IL-2 receptor (IL-2R), IL-4, IL-6, IL-8, IL-10, TNF-α, and INF-γ in the severe patients compared to the non-severe patients (26). Most clinical studies reported that severe COVID-19 patients had significantly higher levels of circulating proinflammatory cytokines/chemokines, including IL-2, IL-2R, IL-6, IL-8, IL-10, and TNF-α, than mild patients (Table 4) (12, 19, 20, 27-33). Our results are consistent with other studies, suggesting that the higher levels of IL-6 and IL-8 are associated with the disease severity.
| Author and Year | Country | Groups | Cases | Age | Sex (male, %) | Methods | Cytokines | Reference |
|---|---|---|---|---|---|---|---|---|
| Liu et al. 2020 | China | Mild | 27 | 43.2 ± 12.3 | 8 (29.6) | Flow cytometry | IL-6, IL-10, IL-2 and IFN-γ | (20) |
| Severe | 13 | 59.7 ± 10.1 | 7 (53.8) | |||||
| Liu et al. 2021 | China | Mild | 46 | NA | NA | NA | IL-2R, IL-6, and IL-8 | (27) |
| Severe | 30 | NA | NA | |||||
| Zhang et al. 2020 | China | Mild | 29 | 44.34 ± 15.84 | 17 (58.6) | Flow cytometry | IL-6 and IL-10 | (28) |
| Severe | 14 | 61.7 ± 9.22 | 5 (35.7) | |||||
| Wan et al. 2020 | China | Mild | 102 | 43.05 ± 13.12 | 55 (53.9) | NA | IL-6 and IL-10 | (29) |
| Severe | 21 | 61.29 ± 15.55 | 11 (52.4) | |||||
| Tan et al. 2020 | China | Mild/moderate | 31 | 44.5 | 17 (53.1) | Flow cytometry | IL-2 and IL-6, IL-10 and TNF-α | (30) |
| Severe | 25 | 66 | 18(72) | |||||
| Chen et al. 2020 | China | Moderate | 10 | 52 | 7 (70.0) | NA | IL-2R, IL-6, IL-10, and TNF-α | (31) |
| Severe | 11 | 61 | 10 (90.9) | |||||
| Li et al. 2020 | China | Non-severe | 279 | 56 | 126 (45.2) | NA | IL-2R, IL-6, IL-10, and TNF-α. | (32) |
| Severe | 269 | 65 | 153 (56.9) | |||||
| Qin et al. 2020 | China | Non-severe | 166 | 53 | 80 (48.2) | NA | IL-2R, IL-6, IL-8, IL-10, and TNF-α | (19) |
| Severe | 286 | 61 | 155 (54.2) | |||||
| Yang et al. 2020 | China | Non-severe | 69 | 42.1 ± 18.6 | 38 (55.1) | Flow cytometry | IL-2R, IL-6, IL-8 and IL-10 | (12) |
| Severe | 24 | 57.9 ± 11.8 | 18 (75) | |||||
| Huang et al. 2020 | China | ICU | 13 | 49 | NA | Multiplex Immunoassay | IL-2, IL-7, IL-10, GCSF, IP-10, MCP1, MIP1A and TNF-α | (33) |
| Non-ICU | 28 | 49 | NA | |||||
| Our study | Turkey | Mild | 32 | 43,1 ± 13,3 | 18 (56.2) | Multiplex Immunoassay | IL-6 and IL-8 | |
| Severe | 46 | 58 ± 15 | 36 (78.3) |
IL-6 is a well-known proinflammatory cytokine induced by endothelial cells myeloid cells, smooth muscle cells, and T cells, and it takes part in different signal transduction pathways (34). The binding of the SARS-COV-2 spike protein to ACE (angiotensin-converting enzyme)-2 as its receptor results in activation of signal transduction pathways leading to production of IL-6 (35). The increased level of IL-6 was found to be associated with ICU admission, ARDS, and death in COVID-19 infection (36). Several studies showed that serum IL-6 levels had a potential prognostic value for need of mechanical ventilation, severity of disease, and mortality in COVID-19 (37). There is insufficient knowledge about the role of chemokines in immunopathogenesis of COVID-19 compared to cytokines, even though they are being reported increasingly.
Previous studies showed that upregulated chemokines, including CXCL8, CXCL10, and CCL2 were correlated with disease severity and increased mortality in COVID-19 patients (38). CXCL8, also known as IL-8, is a potent chemoattractant that recruits and activates neutrophils in the lungs during inflammation, and it may play a major role in development of ARDS in COVID-19 patients (39, 40). The high level of IL-8 has been demonstrated to correlate with increased amounts of neutrophils in the peripheral blood and infiltration of neutrophils into the lungs during COVID-19 infection. Also, Ma et al. reported that the high levels of circulating IL-8 were associated with duration of illness in severe COVID-19 patients (41).
The determination of association between severity of COVID-19 and IL-6 and IL-8 levels provides supportive approach to ongoing clinical trials and studies that are targeting cytokine pathways, such as IL-6 inhibitors like tocilizumab (TCZ), sarilumab, and siltuximab or IL-8 inhibitors like BMS-986253 and reparixin (42-44). Also, interfering with the production of and reducing the levels of these cytokines/chemokines seems to be a promising immunomodulation strategy to control the infection-associated hyperinflammation in severe COVID-19 cases. Xu et al. observed that the symptoms, laboratory abnormalities, and CT opacity changes improved, but oxygen requirement reduced in all patients treated with TCZ (45).
A meta-analysis including 3,641 patients from 16 studies showed that TCZ might reduce mortality in severe patients (46). However, a significant problem that needs to be answered is which therapeutic blockade for COVID-19 treatment should be used since a variety of cytokines and chemokines have been reported to be increased in several studies comparing severe and non-severe cases. Hence, further studies are needed to explore the role of specific immunomodulation with cytokine/chemokine antagonists.
This study had several limitations. First, we could not determine the kinetic changes of inflammatory response in disease progression, as sequential serum samples were not available. Second, we could evaluate only seven biomarkers, although our study was designed to investigate the extensive plasma cytokine and chemokine profiles of severe and mild COVID-19 patients due to low levels that could not be interpreted using standard curves. More extensive studies of cytokine and chemokine profiles may help to understand the immunological mechanisms of COVID-19.
5.1. Conclusions
This is the first clinical study from Turkey descriptively evaluating the cytokine and chemokine levels in COVID-19 patients. Our results demonstrated that IL-6 and IL-8 levels were higher in severe patients. Also, the levels of IL-6 and IL-8 can be used as potential prognostic biomarkers of disease severity in COVID-19 patients. Therefore, detection of plasma cytokine and chemokine levels may be an indicator of disease progression and provide alternative approaches for immunomodulation of severe COVID-19 cases to control the infection-associated hyperinflammation.