1. Background
Hepatitis C is one of the major causes of cirrhosis and hepatocellular carcinoma (HCC) due to chronic liver disease (1, 2). There are about 70 million chronic hepatitis C patients in the world, and since the disease is usually asymptomatic until cirrhosis and HCC develop, there could be delays in diagnosis and treatment (2). Tests used in the diagnosis of hepatitis C fall into two general categories. The first is serological tests that detect antibodies against the hepatitis C virus (HCV), and the second is molecular analyses that detect HCV RNA genomes (3). Anti-hepatitis C virus (anti-HCV) is used as a screening test for hepatitis C and HCV RNA is used as a verification test. Recombinant immunoblot assay (RIBA), which has been used as a validation test until recently, was removed from routine use with the recommendation of Centers for Disease Control and Prevention (CDC) (4).
A false-positive result means that a test has incorrectly detected an illness or disease in someone who does not have the condition. Anti-HCV false positivity can be defined as a positive test result even though patients do not have hepatitis C infection. There is no standard way of distinguishing recovered and current HCV infection from false positivity (5). Anti-HCV is the only screening test used in the diagnosis of hepatitis C. A positive result from this test can be assessed as existing hepatitis C infection, post-treatment remission, past HCV infection, or false positivity (6, 7). A current HCV infection is diagnosed by detecting HCV RNA by polymerase chain reaction (PCR). According to the recommendations of the European Association for the Study of the Liver (EASL), negative HCV-RNA results should be repeated after 12 - 24 weeks to eliminate acute and existing HCV infection (2).
Anti-HCV positivity may be due to chronic hepatitis C treatment or spontaneous viral clearance, as well as false positivity (7). For the definitive diagnosis of hepatitis C infection, the result must be confirmed with HCV RNA (6). The detection of HCV RNA by PCR in patient samples provides evidence of active HCV infection, confirms the diagnosis, and monitors antiviral response to treatment. False anti-HCV positivity is frequent in routine practice and is related to foreign bodies, malaria, biliary cirrhosis, autoimmunity, pregnancy, multiparity, and gender (8-10).
2. Objectives
In this study, we examined anti-HCV positivity rates in our hospital. The aim of administering the anti-HCV tests was to distinguish patients with hepatitis C infection from false positivity in patients with reactive results and to determine the clinical and examination request indications for an anti-HCV request.
3. Methods
3.1. Patients
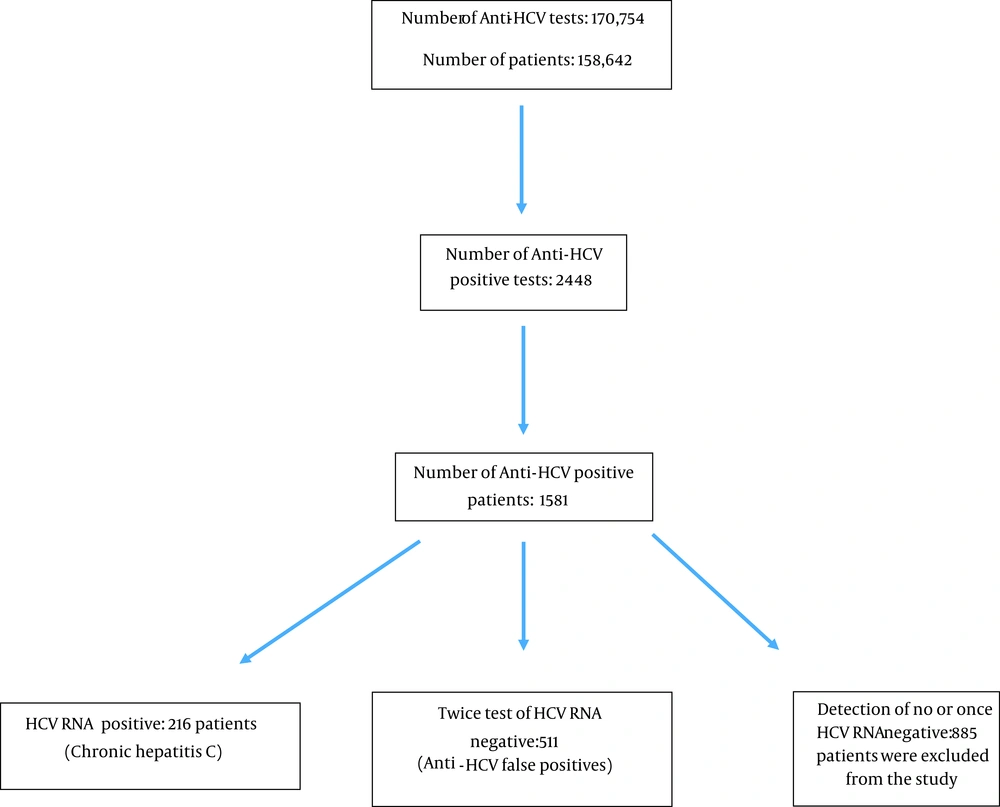
In this cross-sectional study, we included all the anti-HCV tests performed at the Fatih Sultan Mehmet Training and Research Hospital in Istanbul, Turkey, between January 1, 2015, and December 31, 2019. Anti-HCV results were considered positive if signal-to-cutoff ratio (S/Co) ≥ 1 according to the manufacturer’s recommendations (Abbott, USA). During this period, a total of 170,754 anti-HCV tests were performed on a total of 158,642 patients. There were 2448 positive anti-HCV tests. This number dropped to 1,581 after the removal of duplicates, indicating the number of positive patients. The actual number of HCV RNA positive patients was 216 as some patients were double enrolled.
The patient population consisted of a total of 727 patients, including 216 patients with positive HCV-RNA results and 511 patients with false positive results (Figure 1). The patients were retrospectively evaluated in terms of age, gender, anti-HCV titer, the clinic for which examination was requested, the reason for the examination, and the history of hepatitis C. The inclusion criteria were: being over 18 years of age, being anti-HCV positive, and having two negative results from HCV RNA tests performed within an interval of 3 - 6 months. The exclusion criteria were: being under 18 years of age, receiving a reactive anti-HCV result, and not having HCV RNA tested twice. Patients with a history of hepatitis C in their medical records were also excluded from the study.
3.2. Anti-HCV Assays and Quantitative RNA PCR
The anti-HCV test was done on Architect i2000 (Abbott, USA) (chemiluminescent microparticle immunoassay (CMIA) (2nd Generation) device. The Architect Anti-HCV reagent commercial kits were purchased from Abbott Company, Germany. For HCV RNA analysis, Abbott m2000sp was used for isolation and real-time PCR was performed. According to the World Health Organization (WHO) recommendations, the HCV RNA detection limit should be 15 IU/ml. The HCV RNA PCR detection range used in this study was 12-100.000.000 IU/ml, which was very sensitive (Abbott, Germany).
3.3. Definition of False Positivity
In our study, patients who were anti-HCV positive but HCV RNA negative were tested twice with 12 - 24 weeks intervals. Subjects with no history or medical record of recovered HCV infection were considered to have a false positivity for anti-HCV.
3.4. Statistical Analysis
All statistical analyses were performed using SPSS 24.0 statistical software (IBM SPSS Statistics for Windows, NY, USA). T-test was used for continuously changing data, and Chi-square test was used to evaluate discontinuous data. A P-value < 0.05 was considered to be significant in all measurements. Receiver operating characteristic (ROC) analysis was used to calculate the cut-off points by likelihood ratios. Where a significant cut-off value was observed, the sensitivity, specificity, and positive and negative predictive values were presented with 95% confidence interval (CI).
4. Results
In this study, 216 patients with positive results for HCV RNA and 511 patients with negative PCR tests twice were evaluated as false positive cases and included (Table 1). Out of 727 cases, 511 (70.2%) patients were found to have a false positive result. Also, 885 patients who did not have PCR tests for different reasons or who had HCV-RNA negativity were excluded from the study (Figure 1). Genotype analysis was performed on 124 of 216 patients who were found to be positive for HCV RNA; 82% of the cases were Genotype-1, 13% were Genotype-3, and the remaining 5% were other genotypes and mixed genotypes. Anti-HCV titer is related to HCV-RNA positivity (viremia) in the distinction of chronic hepatitis C infection from false positivity (Table 1). The most common indications for anti-HCV examination can be listed as preoperative preparation, pre-immunosuppressive treatment, and being a hemodialysis patient. There were significantly higher rates of HCV-RNA positivity in those requested with suspicion of hepatitis compared to others (P < 0.001) (Table 2). The rate of anti-HCV positivity in internal clinics is considerably higher than in surgical clinics. (P < 0.001) (Table 3).
a Two patients with an anti-HCV titer between 2.1 - 3.0 were drug addicts and were diagnosed with acute hepatitis C. Anti-HCV titers increased during follow-up.
b The lowest anti-HCV titer was 5.2 in HCV-RNA positive patients who did not have an acute hepatitis.
| Suspected Hepatitis | Preoperative Routine Examination | Pre-immuno Suppressive Treatment Routine | Health Worker Routine | Hemodialysis Patient | Healthcare Commission | Total | |
|---|---|---|---|---|---|---|---|
| HCV RNA | |||||||
| Negative | 266 | 161 | 28 | 12 | 15 | 29 | 511 |
| Positive | 172 | 31 | 3 | 1 | 2 | 7 | 216 |
| Total | 438 | 192 | 31 | 13 | 17 | 36 | 727 |
| Internal | Surgical | Total | |
|---|---|---|---|
| HCV RNA | |||
| Negative | 350 | 161 | 511 |
| Positive | 185 | 31 | 216 |
| Total | 535 | 192 | 727 |
5. Discussion
While anti-HCV false negativity is rarely seen in cases of severe immunosuppression and early periods of acute hepatitis C, we frequently encounter false anti-HCV positivity in our daily clinical practice (2-6). Genotype analysis was performed on 124 of 216 patients who were found to be HCV RNA positive; 82% of the cases were determined to be Genotype-1, 13% were Genotype-3, and the remaining 5% were other genotypes and mixed genotypes. The genotype distribution of our study was in line with other studies conducted in Turkey (11). In our study, 70.2% of the patients were found to have a false positive result. In a study by Kirisci et al. in Turkey, the false positivity rate was found to be 61%, which was close to the rates in our study (12). The false positivity rates were found to be 58% and 48.9% in the studies conducted by Aydin et al. and Moorman et al., respectively (13, 14). Similar to other studies, anti-HCV false positivity is high due to the low endemicity for HCV in Turkey.
In the study by Ali and Lal, the false positivity rate was found to be 16.9% (6). It can be argued that the level of false positivity is low in this study since Pakistan is a region of high endemicity for HCV. The false positivity rate was found to be 24.4% in a study conducted in Egypt (15). The reason for these low levels may be that the region is hyperendemic for HCV (prevalence 21 - 24%) and that patient population consists mostly of blood donors. Consistent with CDC data, low false positivity rates are very low in high endemicity regions. In the differentiation of chronic hepatitis C and false positivity, the anti-HCV titer is consistent with the literature findings. The real HCV patients and patients who were evaluated as false positive cases had significantly different anti-HCV titers (Table 1) (13, 16, 17).
At the same time, the probability of HCV infection increased logarithmically, correlating with anti-HCV titer. The lowest anti-HCV titer was found to be 5.2 in patients without acute hepatitis, who were HCV-RNA positive, diagnosed with chronic hepatitis C. Of the 255 cases with anti-HCV < 5, all cases were HCV-RNA negative except two who had acute hepatitis C. Guidelines recommend that HCV-RNA should be checked in acute hepatitis even if anti-HCV is negative (3). When there is a suspicion of acute HCV infection due to known suspicious exposure, clinical picture, or high aminotransferase levels, it is recommended to examine HCV-RNA together with anti-HCV antibodies (3). Therefore, it is thought that increasing the cut-off value will not cause a problem in terms of missing a diagnosis of acute hepatitis C.
In the study by Sayan et al., the lowest anti-HCV titer was found to be 3.8 in patients with HCV infection, but no data about the patients’ clinical condition was reported (16). In the study by Oethinger et al., no patients with anti-HCV < 5 and HCV viremia were found (18). In accordance with the literature, in our study, the relationship between anti-HCV titer and viremia was anti-HCV titer < 5, and no HCV infection was detected when the two patients diagnosed with acute hepatitis C were excluded. In our study, if the anti-HCV titer cut-off value is determined as 5, the sensitivity and specificity would be 99.1% and is 87.9%, respectively. In addition, the cut-off value was determined to be 7.5 (area under the curve [AUC]: 0.982), with the highest sensitivity of 94.4% and specificity of 94.5%.
False positive cases due to an increase in non-specific antibodies may be observed without a history HCV (6). False positivity can be detected in autoimmune diseases such as autoimmune hepatitis, Sjogren’s syndrome, lichen planus, thyroiditis, and polyarteritis nodosa (PAN) (4). Anti-HCV false positivity can also be detected in malaria (9). In another study, 59% of the patients with left ventricular assist device were found to have false positivity and the mean cut-off level was found to be 3.4 (19). In our study, hepatitis C infection was not frequently encountered in these titers. In summary, past infections, rheumatic diseases, and some foreign bodies in the body can be related to false anti-HCV positivity.
The causes of false positivity in our patients could not be examined due to the lack of data. Contrary to the ideas that low-titer anti-HCV positivity without HCV viremia is due to cross-reaction and high-titer anti-HCV positivity is due to a previous asymptomatic hepatitis C infection, the study by Toyoda et al. showed that after hepatitis C treatment, anti-HCV titers decreased overtime (20). Hence, asymptomatic hepatitis C infection and false positivity discrimination in patients with low anti-HCV titers do not seem to be possible with routine examinations. However, HCV viremia is not detected in almost 100% of the patients who do not have clinical, and laboratory signs of hepatitis, and their antibody titers are below 5 S/CO. It may be an appropriate approach for the definition of ‘borderline’ to be reflected in the reporting of the anti-HCV tests like the reports of some other viral serological tests to prevent panic in patients.
In our study, most of the patients were suspected hepatitis cases. Apart from suspected hepatitis, 192 patients were found to be anti-HCV positive in preoperative examinations, of whom 31 patients were diagnosed with chronic hepatitis C after the detection of HCV-RNA positivity. Although hepatitis serology is not recommended in preoperative routine examinations, 31 chronic hepatitis C patients from our hospital’s records were diagnosed and received treatment in earlier stages of the disease thanks to these routine examinations. The routine examination prevented possible liver cirrhosis and hepatocellular carcinoma formation in these patients. Therefore, preoperative routine hepatitis serology examination was important for detecting occult patients. While the CDC previously recommended only screening adults and high-risk individuals born between 1945 and 1965, it now recommends all adults over the age of 18 to be screened for hepatitis C infection (21).
Direct-acting agents (DAA) and more than 90% of the curative treatments have been used in the treatment of chronic hepatitis C in recent years (22). The preoperative routine examinations can detect asymptomatic patients and render early treatment possible. Hence, considering the possible asymptomatic course of the disease, the existence of highly effective treatments, and the reality that routine controls may not always be done in our country, we deem preoperative routine examinations significant. The low rate of anti-HCV positivity in examinations from surgical clinics may be due to routine anti-HCV requests from patients without hepatitis symptoms before surgery. As expected in our study, because requests from internal clinics were made with clinical and laboratory findings of hepatitis, a high PCR positivity rate was found (P < 0.001).
5.1. Conclusions
According to our results, reporting anti-HCV titer of 0 - 5 as negative, 5 - 7.5 as borderline, and > 7.5 as positive might be a more appropriate approach. If there is a clinical suspicion of hepatitis, it should be kept in mind that low antibody titers may be meaningful. Working with a larger number of tests with a more acceptable cut-off level would allow the diagnoses of patients with asymptomatic HCV infection; it can also reduce the cost due to reduced PCR testing requirements.