1. Background
Salmonella is one of the most important foodborne pathogens responsible for an approximately three billion cases of diarrheal diseases each year (1, 2). For non-typhoidal salmonellosis, the mean infective dose to produce symptomatic disease in healthy adults is 105 - 108. A smaller inoculum, on the other hand, can induce infections in newborns and people with specific underlying conditions like human immunodeficiency virus (HIV) infection, cancer, or other immune suppressing conditions (3, 4).
Water and animal-derived foods, such as meat, poultry, eggs, and dairy products, as well as raw fruits and vegetables, can cause non-typhoidal Salmonella infections (5, 6). In contrast to the invasive salmonellosis that requires prompt antibiotic therapy, antibiotics are not usually crucial for the treatment of Salmonella gastroenteritis. Trimethoprim/sulfamethoxazole (SXT), fluoroquinolones, and oxyimino-cephalosporins are commonly recommended as therapeutic options for patients with severe infections (7). Quinolone-resistant Salmonella isolates in people have been reported often in recent years and have become increasingly widespread worldwide (8, 9). Misappropriation of prescribed antibiotics and horizontal gene transfer have been responsible for the increase of Salmonella resistant to conventional antimicrobials (such as quinolones) in Iran (10, 11).
The main chromosomal point mutations occur in topoisomerase IV (parC and parE) and DNA gyrAse (gyrA and gyrB) encoding genes (10, 12). Meanwhile, quinolone resistance has been shown to be caused by changes in efflux pump production and DNA gyrAse protection by the qnr protein, which is created from plasmid-mediated quinolone resistance (PMQR) genes (qnrA, qnrB, qnrS, and aac(6′)Ib-cr) on a conjugative plasmid or transposon (8, 13). Quinolone resistance in foodborne Salmonella could be linked to the occurrence of foodborne outbreaks and the subsequent problem of clinical treatment. Thus, understanding the antibiotic resistance molecular mechanisms, especially quinolones, in Salmonella isolates can be helpful.
2. Objectives
The present study aimed to investigate the occurrence of quinolone resistance among non-typhoidal Salmonella strains isolated from patients in Tehran, Iran, and to elucidate the mechanisms behind it.
3. Methods
The present study involved Salmonella isolates originated from hospitalized individuals and outpatients in Tehran, Iran, based on laboratory confirmation and clinical presentations as described previously (7). A total of 141 Salmonella clinical strains were isolated, among which 113 (80%) strains were collected from stools, and 28 (20%) strains from blood, wound, urine, or other biological fluids. Only one isolate per patient was included in this study. Nalidixic acid-resistant Salmonella isolates were serotyped by slide agglutination method with anti-O and H antisera (Staten Serum Institute, Copenhagen, Denmark). Until use, the validated isolates were kept at -70°C in tryptic soy broth (TSB, Merck KGaA, Darmstadt, Germany) with 25% (v/v) glycerol (14). All ethical issues, including the subjects' health issues, dignity, integrity, right to self-determination, privacy, and confidentiality of personal information were considered.
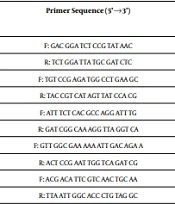
According to the guidelines of the Clinical and Laboratory Standards Institute (CLSI), antimicrobial susceptibility to nalidixic acid (30 μg) and ciprfloxacin (5 μg) was performed on Mueller-Hinton agar (Oxoid Ltd.) using the Kirby-Bauer disk diffusion method (15). As reported previously, the method for DNA extraction was boiling. To amplify the quinolone resistance-determining region (QRDR) of topoisomerase genes gyrA, qnrS, qnrA, and qnrB among the nalidixic acid-resistant strains, the primer sets listed in Table 1 were used. Polymerase chain reaction (PCR) was performed in a volume of 25 µL using a thermal cycler (Eppendorf, Hamburg, Germany) for 30 cycles, as previously described. The cycling conditions were as follows: an initial denaturation at 94°C for 10 minutes followed by denaturation at 94°C for 1 minute, primer annealing at 52 - 60°C for 1 minute, primer extension at 72°C for 1 minute, and the final extension at 72°C for 7 minutes. The PCR products were separated by electrophoresis at 100V for 2 hours on 1.5% (w/v) agarose gels, and visualized using an ultraviolet (UV) transilluminator (Tanon, Shanghai, China).
| Gene | Primer | Primer Sequence (5'→3') | Amplicon Size (bp) | Annealing Temperature (°C) | Reference |
|---|---|---|---|---|---|
| gyrA | DsgyrA | F: GAC GGA TCT CCG TAT AAC | 317 | 52 | This study |
| R: TCT GGA TTA TGC GAT CTC | |||||
| gyrA | LsgyrA | F: TGT CCG AGA TGG CCT GAA GC | 347 | 60 | (16) |
| R: TAC CGT CAT AGT TAT CCA CG | |||||
| qnrA | DsqnrA | F: ATT TCT CAC GCC AGG ATT TG | 516 | 55 | (17) |
| R: GAT CGG CAA AGG TTA GGT CA | |||||
| qnrB | DsqnrB | F: GTT GGC GAA AAA ATT GAC AGA A | 500 | 52 | (18) |
| R: ACT CCG AAT TGG TCA GAT CG | |||||
| qnrS | DsqnrS | F: ACG ACA TTC GTC AAC TGC AA | 417 | 55 | (18) |
| R: TTA ATT GGC ACC CTG TAG GC |
PCR Primers Used in This Study
Restriction fragment length polymorphism (RFLP) was performed to determine the likely mutation in the gyrA gene among nalidixic acid-resistant strains. Briefly, 12 µL of PCR-amplified fragment was digested with 1 µL of HinfI (MBI Fermentas) in 3 µL digestion buffer in a final volume of 20 µL, and incubated at 37°C. After overnight incubation at 37°C, restriction fragments were subjected to 2% agarose gel electrophoresis, stained with ethidium bromide, and examined by UV transillumination before being photographed. To identify mutant isolates, PCR products were sequenced immediately (Pishgam co., Tehran, Iran). The DNA sequences of the mutant strains were deposited into the GenBank database (ncbi.nlm.nih.gov) under accession numbers KF975705, KF975707, KF975709, KF975710, and KF975711. As shown in Figure 1, nucleotide sequences obtained from sequencing analysis were translated into amino acid sequences and aligned by the Multiple Alignment using Fast Fourier Transform (MAFFT) program (mafft.cbrc.jp).
4. Results
Amongst the 141 Salmonella isolates, 60% were nalidixic acid-resistant strains. However, none of them were resistant to ciprofloxacin or showed reduced susceptibility to it. Also, a total of seven different serotypes were identified. The commonly prevalent serotypes were S. Enteritidis and S. Infantis (Table 2). According to our results, 17 (20%) of the 85 nalidixic acid-resistant strains carried the qnrS gene, all of which belonged to the Enteritidis serotype. Furthermore, the qnrA and qnrB genes were not found in nalidixic acid-resistant strains.
| Salmonella Serotype | Nalidixic Acid Resistant Isolates, No. (%) |
|---|---|
| Enteritidis | 39 (45.88) |
| Infantis | 35 (41.18) |
| Albany | 4 (4.71) |
| Hadar | 2 (2.35) |
| Munchen | 2 (2.35) |
| Typhimurium | 2 (2.35) |
| Haifa | 1 (1.17) |
| Total | 85 (100) |
Distribution of Serotypes Among Nalidixic Acid-Resistant Salmonella Isolates
Non-mutant and mutant PCR-amplified gyrA fragments have three and two HinfI restriction sites, respectively (Table 3). Restriction analysis of the PCR product revealed that 16 (18.8%) isolates showed mutant pattern. Sequencing of gyrA PCR products was used to confirm mutations associated with nalidixic acid resistance in 16 resistant organisms that displayed two bands in PCR-RFLP. Nucleotide sequencing showed that 16 isolates had mutations in gyrA in terms of change of the amino acid serine to phenylalanine (Ser83→Phe, 7 isolates), serine to tyrosine (Ser83→Tyr, 5 isolates), aspartate to tyrosine (Asp87→Tyr, 3 isolates), and proline to leucine (Pro43→Leu, 1 isolate) (Figure 1). Amongst the seven isolates containing the substitution Ser83→Phe in gyrA, one isolate had multiple mutations (Ala51→Thr, Ser83→Phe, Val85→Leu, Ile89→Leu, Val90→Asp, Ala93→Ser, Gln94→His, Gly110→Arg, Ala117→Thr, and Ala118→Pro). Furthermore, two isolates harbored mutation at position 83 (Ser83→Phe) and carried qnrS gene (GenBank Accession Numbers: KF975711.1, KF975710.1, KF975709.1, KF975708.1, KF975707.1, KF975706.1, KF975705.1, KF975704.1, KC470542.1).
| Gene and Primer | Amplicon Size (bp) | Position of Restriction Sites | Restriction Fragment Length (bp) | |
|---|---|---|---|---|
| Non-mutant | Mutant | |||
| gyrA/ LsgyrA | 347 | 108 and 207 | 108, 99, and 140 | 108 and 239 or 207 and 140 |
| gyrA/ DsgyrA | 317 | 33 and 132 | 33,99 and 185 | 33 and 284 or 132 and 185 |
HinfI Restriction Pattern and the Length of Resulting Fragments
5. Discussion
Salmonella infections are common in many developing nations, including Iran. They rarely appear as a severe public health hazard in the industrialized countries. In previous investigations conducted in Iran, S. enterica serotype Enteritidis was found to be the most common serotype of Salmonella in people. Moreover, in a comprehensive study conducted in China, United States, and Taiwan, S. enterica serotype Enteritidis was the most prevalent strain serotype among human isolates (19-22). These results are in agreement with our results, which revealed that S. enterica serotype Enteritidis was the predominant serotype. However, other studies in Ghana, Armenia, and Georgia reported S. enterica serotype Typhimurium as the main serotype (14, 23). In humans, S. Enteritidis infections are most usually linked to contaminated chicken and its products, whereas S. Typhimurium infections are mostly linked to infected pig and bovine derivatives (2). Local agriculture and farming practices, food distribution and consumption patterns, as well as food consumer preferences and behaviors could play a role in Salmonella serotype ranking in a well-defined geographical area like Tehran, Iran.
Over the past decade, since these organisms have been linked to clinical failures of therapy and a considerable burden of hospitalization, the high incidence of antimicrobial-resistant Salmonella isolates has been a critical public health concern (24-26). Resistance to nalidixic acid, for example, is a concerning scenario because fluoroquinolones are the most often used antibiotics for the treatment of invasive salmonellosis in adults, and failure of therapy in individuals with nalidixic acid-resistant Salmonella infections has been observed (27). In Iran, resistance to nalidixic acid has been increased among non-typhoidal S. enterica isolates (27), as in our study, 60% of isolates were resistant to nalidixic acid. In a previous study in Iran, a high level of resistance to nalidixic acid among Salmonella strains has been reported (28). In this regard, a meta–analysis study investigated fluoroquinolone-resistant clinical strains in Iran. According to their results, the pooled prevalence of nalidixic acid-resistant isolates in Iran was 48.1%. Moreover, from 1983 to 2019, the nalidixic acid-resistance trend of Salmonella serotypes was increasing in Iran (11).
In contrast, our results regarding the resistance to nalidixic acid in human isolates of S. Enteritidis were higher than those of South East Asian countries, including Malaysia, Thailand, and Vietnam (27 - 38%) (29-31). In addition to nalidixic acid, no ciprfloxacin-resistant isolate was found in the present study. This finding is in line with the current situation in Tehran, where no ciprofloxacin-resistant isolates were detected among 174 S. enterica strains (32). However, a comprehensive analysis by Khademi et al. revealed that the pooled occurrence of ciprofloxacin-resistant Salmonella serotypes in clinical specimens was 2.9% in Iran (11). In Salmonella spp., point mutations at QRDR of DNA gyrAse are mainly the cause of quinolone resistance between amino acids 67 and 106, which can alter the binding site of the agents with DNA gyrAse (33). Single mutations in the gyrA gene have been discovered to be sufficient to provide substantial levels of resistance to quinolones like nalidixic acid in Salmonella spp. (34).
In the present study, genetic characterization of nalidixic acid resistance revealed that all mutant S. enterica isolates had point mutations at codons 83 or 87 of GyrA, except one isolate that had point mutation at position 43 (Pro43→Leu), which was not previously reported. Double substitutions at both positions 83 and 87 were not identified in this study. The most common point mutation of gyrA occurred at position 83 (Ser→Phe, 43.7%). In contrast to our finding, previous reports from Iran showed that the predominant substitution was at position 87 (Asp87→Asn) of GyrA (32, 35). In agreement with the current study, the highest prevalence of amino acid substitution in GyrA was Ser83→Phe (92.1%) among nalidixic acid-resistant S. enterica serotype Typhi strains, isolated from southern Vietnam (36). Indeed, these reports from different countries indicate that the presence of mutation at Ser83 may cause reduced susceptibility to ciprofloxacin, representing high level of nalidixic acid resistance (36).
Plasmid-borne resistance, such as PMQR in S. enterica, is a public health concern because it results in horizontal fluoroquinolone resistance transfer between strains (37). In this study, 20% of isolates harbored the qnrS gene. Moreover, PCR analysis of the quinolone-resistant strains did not detect qnrA or qnrB genes. These findings partly agree with previous studies conducted in Korea and United States (10, 38). There are few studies investigating the PMQR in non-typhoidal Salmonella isolates in Iran. However, a recent study by Saboohi et al. in Iran demonstrated that 25.8%, 1.17%, and 1.17% of Salmonella spp. harbored qnrA, qnrB, and qnrS genes, respectively (39). Contrary to our finding, Abbasi and Ghaznavi-Rad revealed that among the PMQR genes, qnrS, qnrA, and qnrB were positive in 60%, 40%, and 20% of the isolates, respectively (28).
5.1. Conclusions
This study provided an insight into the molecular mechanism of quinolone resistance in non-typhoidal Salmonella strains isolated from patients in Tehran, Iran. We cannot rule out the presence of mutations outside of the sequenced region, but our findings suggest that other possible mechanisms may play a role regarding the quinolone resistance in Salmonella isolates. These mechanisms could include parE mutations, changes in expression of eflux pumps, modifications of the outer membrane proteins or even novel mechanisms. Further studies are required to monitor the spread of non-typhoidal Salmonella involving QRDR and PMQR carriers and to determine other mechanisms of quinolone resistance.