1. Background
In recent years, Candida species has emerged as one of the most common causes of bloodstream infections (BSIs) (1-5). Candidemia, known as Candida BSI, is mostly associated with intra-abdominal surgical procedures, long-term and broad-spectrum antibiotics, intravenous devices, immunosuppressive drugs, and total parenteral nutrition (6, 7). Candidemia is usually diagnosed using blood cultures; however, blood culture positivity can be detected in nearly half of invasive Candida infections (8). In this limited diagnosis, the true epidemiology of candidemia is unclear and may vary. The distribution of Candida spp. that causes candidemia varies according to different geographical areas and even hospital units. This difference in the distribution of Candida spp. is due to predisposing conditions in patients, hospital-related factors, and antifungal drug exposure (9, 10).
The incidence of candidemia has been changing in most regions of the world with the emergence of non-albicans Candida (NAC) species (2, 7). This change and, especially, the increasing use of azole antifungal agents have brought forth antifungal resistance and treatment difficulty. There are limited data on the incidence of candidemia in Turkey and was reported 1.23 to 13.3 episodes/1000 admissions, respectively (11, 12). Furthermore, there are limited data about the distribution of Candida spp. and susceptibility by year.
2. Objectives
In this study, we aimed to analyze changes in the distribution of Candida spp. and their antifungal susceptibility profiles from blood cultures during the 2016 - 2020 period at a tertiary care center in Istanbul, Turkey.
3. Methods
This single-center retrospective study was performed on positive blood cultures for Candida spp. between January 2016 and December 2020 in a tertiary care center with a capacity of 500 beds in Istanbul, Turkey. This medical center has 4 main intensive care units (ICUs, including neonatal, pediatric, coronary, and cardiovascular surgical care units), 6 surgery departments, and adult and pediatric hematology-oncology departments [including a hematopoietic stem cell transplantation (HSCT) unit].
Candidemia was defined as the first positive culture for Candida growth on blood culture. Only the initial episode from each patient was submitted for evaluation. Blood cultures were monitored by BacT/Alert 3D automated blood culture system (bioMérieux, France), and positive ones were subcultured on Sabouraud dextrose agar (bioMérieux, France) and blood agar plates (bioMérieux, France). Suspected colonies of yeasts were identified, and their antifungal susceptibility tests were performed by the VITEK 2 compact system (bioMérieux, France) using VITEK 2 YST-ID and YST-YS07 cards. We used the VITEK 2 system that concluded an excellent quantitative and qualitative agreement with CLSI and EUCAST broth microdilution reference methods, albeit this is not a comparison study (13, 14). The results of antifungal susceptibility tests were properly evaluated in accordance with the European Committee on Antimicrobial Susceptibility Testing (15). Fluconazole, voriconazole, amphotericin B, caspofungin, and micafungin antifungal susceptibility results were evaluated. Minimal inhibitory concentrations (MIC50 and MIC90) and MIC geometric mean (MIC GM) assessment were evaluated when at least 10 Candida spp. were detected.
3.1. Statistical Analysis
Statistical analyses were performed using SPSS version 15.0 (SPSS, Chicago, IL, USA). Categorical variables were reported as numbers and percentages, while numerical variables were reported as mean ± SD, minimum and maximum. The analyses were performed using the chi-square test. P values < 0.05 were considered statistically significant.
4. Results
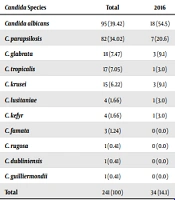
Of the initial 360 incidences of Candida BSI, 241 were evaluated. A total of 5 Candida spp., including 3 C.famata, 1 C. guilliermondii, and 1 C. dubliniensis, were isolated. Susceptibility tests were not performed, and the remaining 236 Candida samples were analyzed on the BacT/ALERT 3D system. During the observation period, a total of 11 different Candida spp. were found. Candida albicans was the predominant pathogen (n = 95, 39.42%), followed by C. parapsilosis (n = 82, 34.02%), C. glabrata (n = 18, 7.47%), C. tropicalis (n = 17, 7.05%), C. krusei (n = 15, 6.22%), and other Candida spp. (n = 14, 5.79%). While the ratio of C. albicans decreased from 54.5 to 28.8% between 2016 and 2019, the percentage of it increased to 38,9% in 2020. The ratio of C. parapsilosis tended to increase from 20.6 to 38.9% from 2016 to 2020. There was no statistically significant difference between the percentages of incidents of Candida spp. The distribution of Candida spp. is shown in Table 1.
| Candida Species | Total | 2016 | 2017 | 2018 | 2019 | 2020 | P Value b |
|---|---|---|---|---|---|---|---|
| Candida albicans | 95 (39.42) | 18 (54.5) | 18 (40.9) | 28 (41.2) | 17 (28.8) | 14 (38.9) | 0.195 |
| C. parapsilosis | 82 (34.02) | 7 (20.6) | 13 (29.5) | 26 (38.2) | 22 (37.3) | 14 (38.9) | 0.364 |
| C. glabrata | 18 (7.47) | 3 (9.1) | 3 (6.8) | 3 (4.4) | 7 (11.9) | 2 (5.6) | 0.596 |
| C. tropicalis | 17 (7.05) | 1 (3.0) | 4 (9.1) | 5 (7.4) | 5 (8.5) | 2 (5.6) | 0.875 |
| C. krusei | 15 (6.22) | 3 (9.1) | 1 (2.3) | 3 (4.4) | 5 (8.5) | 3 (8.3) | 0.549 |
| C. lusitaniae | 4 (1.66) | 1 (3.0) | 1 (2.3) | 1 (1.5) | 1 (1.7) | 0 (0.0) | 0.909 |
| C. kefyr | 4 (1.66) | 1 (3.0) | 2 (4.5) | 1 (1.5) | 0 (0.0) | 0 (0.0) | 0.337 |
| C. famata | 3 (1.24) | 0 (0.0) | 1 (2.3) | 1 (1.5) | 0 (0.0) | 1 (2.8) | 0.688 |
| C. rugosa | 1 (0.41) | 0 (0.0) | 1 (2.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.473 |
| C. dubliniensis | 1 (0.41) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0.707 |
| C. guilliermondii | 1 (0.41) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0.707 |
| Total | 241 (100) | 34 (14.1) | 44 (18.3) | 68 (28.3) | 59 (24.5) | 36 (14.9) |
a Values are expressed as No. (%).
b P < 0.05 is statistically significant.
The incidents represent 124 ICU patients (51.25%), 53 pediatric ICU patients (22.08%), 30 patients (12.5%) from the hematology unit, 23 patients (9.58%) from the internal medicine unit, and 11 patients (4.58%) from surgical units. The distribution of Candida spp., by hospital unit, is demonstrated in Table 2. Candida parapsilosis was significantly more common in the pediatric ICU (P < 0.05). Candida albicans was the most common species in the ICU and hematology and surgical clinics, and C. parapsilosis was predominant in internal medicine clinics (P > 0.05). Over 5 years, susceptibility to amphotericin B, fluconazole, voriconazole, caspofungin, and micafungin were determined against the 5 most isolated Candida spp.
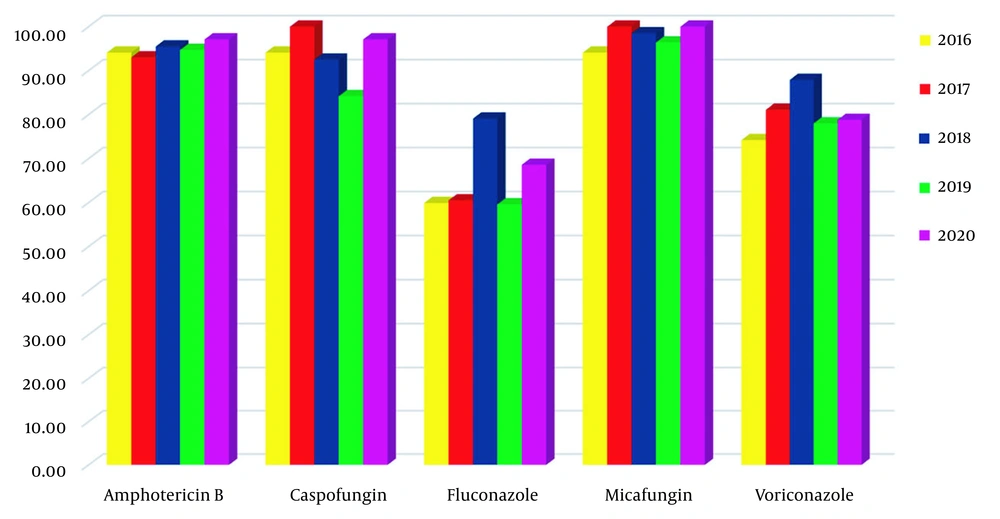
Micafungin susceptibility was the highest with a 97.4% ratio, and fluconazole was the lowest with a 66.1% ratio in 236 isolates. Antifungal susceptibility results and MIC values are shown in Table 3. We found that amphotericin B, caspofungin, and micafungin MIC50 and MIC90 values were low for many Candida spp., while these values were high for C. krusei. Reduced sensitivities to fluconazole and voriconazole for C. albicans and C. parapsilosis were found. None of the C. glabrata isolates were sensitive to fluconazole. Over the years, the change in susceptibility ratio was statistically significant for fluconazole toward C. albicans, as well as for voriconazole toward both C. albicans (P < 0.005) and C. parapsilosis. Besides, the evaluation of different distribution, caspofungin, and fluconazole sensitivities was statistically significant for all Candida spp (Table 4 and Figure 1).
| Candida Species | Total | ICU | Adult and Pediatric Hematology and Transplantation | Pediatric ICU | Surgical | Internal Medicine | P Value b |
|---|---|---|---|---|---|---|---|
| Candida albicans | 95 (39.42) | 50 (40.3) | 13 (43.3) | 17 (32.1) | 6 (54.5) | 9 (39.1) | 0.647 |
| C. parapsilosis | 82 (34.02) | 42 (33.9) | 4 (13.3) | 25 (47.2) | 1 (9.1) | 10 (43.5) | 0.008 |
| C. glabrata | 18 (7.47) | 8 (6.5) | 4 (13.3) | 3 (5.7) | 3 (27.3) | 0 (0.0) | 0.055 |
| C. tropicalis | 17 (7.05) | 9 (7.3) | 3 (10.0) | 2 (3.8) | 0 (0.0) | 3 (13.0) | 0.518 |
| C. krusei | 15 (6.22) | 6 (4.8) | 4 (13.3) | 4 (7.5) | 1 (9.1) | 0 (0.0) | 0.240 |
| Others c | 14 (5.81) | 9 (7.3) | 2 (6.7) | 2 (3.8) | 0 (0.0) | 1 (4.3) | 0.919 |
| Total | 241 (100) | 124 (51.25) | 30 (12.5) | 53 (22.08) | 11 (4.58) | 23 (9.58) |
a Values are expressed as No. (%).
b P < 0.05 is statistically significant.
c Other Candida species are C. lusitaniae, C. kefyr, C. famata, C. rugosa, C. dubliniensis, and C. guilliermondii.
| Candida Species and Antifungal Agent | Minimum Inhibitory Concentration a | In vitro Susceptibility; No. (%) | |||||
|---|---|---|---|---|---|---|---|
| Range (mg/L) | 50% | 90% | GM | S | DDS | R | |
| Candida albicans (n = 95) | |||||||
| AB | ≤ 0.25 - 1 | 0.50 | 1 | 0.49 | 95 (100) | 0 | 0 |
| CAS | ≤ 0.25 - 1 | ≤ 0.12 | ≤ 0.25 | 0.17 | 95 (100) | 0 | 0 |
| FLZ | ≤ 0.5 - 32 | ≤ 0.5 | 16 | 1.15 | 77 (81) | 4 (4.2) | 14 (14.8) |
| MCF | ≤ 0.06 - 0.5 | ≤ 0.06 | ≤ 0.06 | 0.06 | 95 (100) | 0 | 0 |
| VRC | ≤ 0.12 - ≥8 | ≤ 0.12 | 1 | 0.98 | 75 (79) | 2 (2.1) | 18 (18.9) |
| C. parapsilosis (n = 82) | |||||||
| AB | ≤ 0.25 - 8 | 0.50 | 1 | 0.35 | 76 (92.7) | 0 | 6 (7.3) |
| CAS | ≤ 0.25 - 2 | ≤ 0.12 | ≤ 0.25 | 0.71 | 82 (100) | 0 | 0 |
| FLZ | ≤ 0.5 - 32 | 1.00 | 8.00 | 1.78 | 55 (67.1) | 9 (10.9) | 18 (21) |
| MCF | ≤ 0.06 - 2 | 0.50 | 1.00 | 0.54 | 80 (97.6) | 0 | 2 (2.4) |
| VRC | ≤ 0.12 - 2 | ≤ 0.12 | 0.50 | 0.17 | 63 (76.8) | 16 (19.5) | 3 (3.7) |
| C. glabrata (n = 18) | |||||||
| AB | ≤ 0.25 - 8 | 0.50 | 1.00 | 0.56 | 17 (94.4) | 0 | 1 (5.6) |
| CAS | ≤ 0.25 - 0.5 | ≤ 0.12 | 0.25 | 0.23 | 10 (55.6) | 0 | 8 (44.4) |
| FLZ | 2 - 16 | 4.00 | 8.00 | 4.76 | 0 | 17 (94.4) | 1 (5.6) |
| MCF | ≤ 0.06 | ≤ 0.06 | ≤ 0.06 | 0.06 | 18 (100) | 0 | 0 |
| VRC | IE | IE | IE | IE | IE | IE | IE |
| C. tropicalis (n = 17) | |||||||
| AB | ≤ 0.2 - 0.5 | ≤ 0.25 | 0.50 | 0.28 | 17 (100) | 0 | 0 |
| CAS | ≤ 0.12 - 0.25 | ≤ 0.12 | ≤ 0.12 | 0.13 | 17 (100) | 0 | 0 |
| FLZ | ≤ 1 - 16 | 1 | 2 | 1.38 | 15 (88.2) | 0 | 2 (11.8) |
| MCF | ≤ 0.06 | ≤ 0.06 | ≤ 0.06 | 1.11 | 17 (100) | 0 | 0 |
| VRC | ≤ 0.12 - 0.25 | ≤ 0.12 | ≤ 0.12 | 0.12 | 16 (94.1) | 0 | 1 (5.9) |
| C. krusei (n = 15) | |||||||
| AB | ≤ 0.25 - 4 | 0.50 | 4 | 0.78 | 11 (73.4) | 0 | 4 (226.6) |
| CAS | ≤ 0.12 - ≥ 8 | ≤ 0.12 | ≥8 | 0.70 | 8 (53.3) | 0 | 7 (46.7) |
| FLZ | 1 - 32 | 8 | 32 | 6.6 | 0 | 0 | 15 (100) |
| MCF | ≤ 0.06 - ≥ 8 | 0.12 | ≥8 | 1.4 | 12 (80) | 0 | 3 (20) |
| VRC | ≤ 0.12 - 0.25 | ≤ 0.12 | ≤ 0.12 | 0.13 | 15 (100) | 0 | 0 |
| C. kefyr (n = 4) | |||||||
| AB | 1 - 2 | * | * | * | 2 (50) | 0 | 2 (50) |
| CAS | ≤ 0.12 - ≤ 0.25 | * | * | * | 4 (100) | 0 | 0 |
| FLZ | ≤ 0.5 - 2 | * | * | * | 4 (100) | 0 | 0 |
| MCF | 0.12 | * | * | * | 4 (100) | 0 | 0 |
| VRC | ≤ 0.12 | * | * | * | 4 (100) | 0 | 0 |
| C. lusitaniae (n = 4) | |||||||
| AB | 0.5 | * | * | * | 4 (100) | 0 | 0 |
| CAS | ≤ 0.25 - 4 | * | * | * | 3 (75) | 0 | 1 (25) |
| FLZ | ≤ 0.5 - ≤ 1 | * | * | * | 4 (100) | 0 | 0 |
| MCF | 0.12 - 1 | * | * | * | 4 (100) | 0 | 0 |
| VRC | ≤ 0.12 | * | * | * | 4 (100) | 0 | 0 |
| C. rugosa (n = 1) | |||||||
| AB | 0.5 | * | * | * | 1 (100) | 0 | 0 |
| CAS | 1 | * | * | * | 1 (100) | 0 | 0 |
| FLZ | 4 | * | * | * | 1 (100) | 0 | 0 |
| MCF | 0.12 | * | * | * | 1 (100) | 0 | 0 |
| VRC | ≤ 0.12 | * | * | * | 1 (100) | 0 | 0 |
Abbreviations: IE, insufficient evidence; S, susceptible; R, resistant; DDS, dose-dependent susceptible; AMB, amphotericin B; CAS, caspofungin; FCZ, fluconazole; MCF, micafungin; VOR, voriconazole; MIC, minimum inhibitory concentration; GM, geometric mean.
a * MIC50, MIC90, and GM values were not performed because the number is smaller than 10.
| Candida Species and Years | AMB | CAS | FLU | MCF | VRC |
|---|---|---|---|---|---|
| C. albicans (n = 95) | |||||
| 2016 | 18 (100) | 18 (100) | 9 (50) | 18 (100) | 10 (55.6) |
| 2017 | 18 (100) | 18 (100) | 11 (61.1) | 18 (100) | 13 (72.2) |
| 2018 | 28 (100) | 28 (100) | 26 (92.9) | 28 (100) | 25 (89.3) |
| 2019 | 17 (100) | 17 (100) | 16 (94.1) | 16 (94.1) | 16 (94.1) |
| 2020 | 14 (100) | 14 (100) | 14 (100) | 14 (100) | 11 (78.6) |
| P value | - | - | < 0.001 | 0.482 | 0.034 |
| C. parapsilosis (n = 82) | |||||
| 2016 | 7 (100) | 7 (100) | 6 (85.7) | 6 (85.7) | 7 (100) |
| 2017 | 12 (92.3) | 13 (100) | 8 (61.5) | 13 (100) | 11 (84.6) |
| 2018 | 25 (96.2) | 26 (100) | 20 (76.9) | 26 (100) | 23 (88.5) |
| 2019 | 20 (90.9) | 22 (100) | 13 (59.1) | 21 (95.5) | 12 (54.5) |
| 2020 | 13 (92.8) | 14 (100) | 8 (57.1) | 14 (100) | 10 (71.4) |
| P value | 0.913 | - | 0.477 | 0.166 | 0.034 |
| C. glabrata (n = 18) | |||||
| 2016 | 3 (100) | 3 (100) | 0 | 3 (100) | 0 |
| 2017 | 3 (100) | 3 (100) | 0 | 3 (100) | 0 |
| 2018 | 3 (100) | 1 (33.3) | 0 | 3 (100) | 0 |
| 2019 | 6 (85.7) | 2 (28.6) | 0 | 7 (100) | 0 |
| 2020 | 2 (100) | 1 (50) | 0 | 2 (100) | 0 |
| P value | 1.000 | 0.125 | - | - | - |
| C. tropicalis (n = 17) | |||||
| 2016 | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) |
| 2017 | 4 (100) | 4 (100) | 3 (75) | 4 (100) | 3 (75) |
| 2018 | 5 (100) | 5 (100) | 5 (100) | 5 (100) | 5 (100) |
| 2019 | 5 (100) | 5 (100) | 4 (80) | 5 (100) | 5 (100) |
| 2020 | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) |
| P value | - | - | 0.787 | - | 0.378 |
| C. krusei (n = 15) | |||||
| 2016 | 2 (66.7) | 2 (66.7) | 0 | 2 (66.7) | 3 (100) |
| 2017 | 0 | 1 (100) | 0 | 1 (100) | 1 (100) |
| 2018 | 1 (33.3) | 0 | 0 | 2 (66.7) | 3 (100) |
| 2019 | 5 (100) | 1 (20) | 0 | 4 (80) | 5 (100) |
| 2020 | 3 (100) | 3 (100) | 0 | 3 (100) | 3 (100) |
| P value | 0.093 | 0.105 | - | 1.000 | 1.000 |
| All species (n = 236) | |||||
| 2016 | 32 (94.1) | 33 (94.1) | 18 (59.9) | 32 (94.1) | 23 (74.2) |
| 2017 | 40 (93) | 43 (100) | 26 (60.5) | 43 (100) | 30 (81.1) |
| 2018 | 64 (95.5) | 62 (92.5) | 53 (79.1) | 66 (98.5) | 58 (87.9) |
| 2019 | 54 (94.7) | 48 (84.2) | 34 (59.6) | 54 (96.4) | 39 (78) |
| 2020 | 34 (97.1) | 34 (97.1) | 24 (68.6) | 35 (100) | 26 (78.8) |
| Total | 224 (94.9) | 221 (93.6) | 155 (65.7) | 230 (97.4) | 176 (81.1) |
| P value | 1.000 | 0.035 | 0.048 | 0.305 | 0.074 |
Abbreviations: AMB, amphotericin B; CAS, caspofungin; FCZ, fluconazole; MCF, micafungin; VOR, voriconazole.
a Values are expressed as No. (%).
5. Discussion
Incidences of candidemia and the distribution of Candida spp. vary geographically and among different populations, age groups, study periods, types of hospitals, and even hospital units. The distribution of Candida spp. was shifted from C. albicans to NAC species (3, 5, 16-22). When risk factors between C. albicans and NAC BSI were compared, older age and underlying cardiovascular diseases were risk factors for C. albicans, while cancer and chemotherapy were risk factors for NAC (23). Although some researchers have reported no change in the distribution of species over time (24, 25), recent studies from Kuwait (3), Lebanon (17), Italy (18), Israel (19), India (20), Saudi Arabia (21), and China (22) have indicated a predominance of NAC species compared to C. albicans. In this study, the increase in the prevalence of NAC species can be interpreted as the improvement of diagnostic methods and the treatment of cancer patients in our hospital, which has increased in recent years.
Candida albicans, as the most frequently isolated species worldwide (1, 3, 5-7, 11, 16, 18-20, 22, 24-28), was also the leading species isolated in this study (39.4%), followed by C. parapsilosis, C. glabrata, and C. tropicalis (34, 7.4, and 7%, respectively). According to the studies conducted in Turkey, the most frequent species were C. albicans, C. parapsilosis, C. tropicalis, and C. glabrata (7, 11, 27, 29, 30), which is in line with other studies in the literature (6, 16, 17, 21, 22, 24, 26). Candida parapsilosis was reportedly the most frequent species in some studies (27, 31, 32), whereas C. glabrata was the leading species in the studies of Israel et al. (19) and Aldardeer et al. (33). The prevalence of isolated Candida spp. varies among specific age groups. Although Cleveland et al. (4) reported a significant decline in infants and pediatric patients due to the increased use of azoles as prophylaxis in neonatal ICUs, non-compliance with precautionary infection control and increased risk factors (such as diabetes mellitus, ICU admissions, and immunosuppressive treatments) indicated that the prevalence of candidemia in pediatric patients is still high. While C. glabrata was commonly observed in elderly patients (1), C. parapsilosis was found mainly in children and neonates (1, 18, 21). In contrast with the study conducted by Aslan et al. (34), who reported that 42.2% of the pediatric ICU patients had C. albicans, C. parapsilosis was the most common species in our pediatric ICU patients.
The distribution of Candida spp. differs among various departments. Li et al. (5) observed that C. albicans was detected in 51.4% and C. tropicalis in 8.6% of the surgical patients in their facility, whereas C. tropicalis accounted for 27.3% of all cases. Similarly, Israel et al. (19) reported that the leading species was C. tropicalis in hematology-oncology patients and one-third of candidemias occurred in ICUs. Caggiano et al. (18) indicated that C. parapsilosis was most frequently detected in adult and pediatric hematology-oncology patients, and 31.2% of the patients with candidemia were in ICUs, predominantly in the neonatal ICU. In our study, 1.25% of patients were in the ICU and commonly had C. albicans, similar to the rate reported by Ergon et al. in Turkey (25). According to the period analyzed in this study, when the overall prevalence of Candida spp. was evaluated, it was observed that the prevalence of C. albicans (the predominant species) decreased gradually and equalized with C. parapsilosis in 2020. Khan et al. (3) stated that C. albicans was the most frequently isolated species during the period 2006 - 2012 in Kuwait and decreased with an increase in the prevalence of C. parapsilosis, in contrast with the results of Mete et al. (12), who mentioned no significant change in the distribution of the species over time.
The frequency of C. albicans, which had a decreasing trend until 2019, increased again during the COVID-19 pandemic. However, the increase in the frequency of C. parapsilosis in NAC species continued. It has been shown that the use of broad-spectrum antibiotics, the presence or prolonged use of central venous catheters, and immunosuppressive treatments increase fungal infections in the COVID-19 period (35, 36).
Likewise, advanced age and cardiovascular diseases are important risk factors for hospitalization in COVID 19, and immunosuppressive treatments used in the treatment of COVID 19 explain the increase in the frequency of C. albicans that we detected during the pandemic period (37, 38). The prevalence of C. krusei has been reported to be increasing in recent years (2). In this study, 6.22% of our patients had C. krusei candidemia, and no statistically significant differences were noted by year. These epidemiological differences may be related to geographical features, as well as the fact that patients in these studies belong to different risk factors, such as malignancy, exposure to antifungal agents, previous antibiotic use, and the presence of a central venous catheter (9, 10).
Reduced susceptibility to fluconazole has been reported for C. glabrata (1), C. parapsilosis (12), C. tropicalis (16), and C. albicans (17, 20), whereas high susceptibility to fluconazole has been reported for C. albicans (5, 7, 16, 19, 21, 24, 26, 28), C. parapsilosis (7, 21, 24, 26), and C. glabrata (7, 28). High susceptibility rates have been reported for voriconazole in Candida isolates (2, 26, 28). In this study, C. parapsilosis and C. glabrata presented a decrease in susceptibility to fluconazole, whereas C. albicans showed an increased susceptibility. It is known that long-term use of fluconazole plays a role in the development of fluconazole resistance in Candida spp. (39). However, in our hospital, a change from fluconazole to echinocandins had begun in the empirical treatment of candidemia, according to the 2016 Infectious Diseases Society of America (IDSA) guidelines (40). In the current study, an increase in fluconazole and voriconazole sensitivity in C. albicans was interpreted as a result of using echinocandin rather than fluconazole in the empirical treatment option.
Candida parapsilosis has emerged as an important nosocomial pathogen (41). In the current study, the sensitivity of fluconazole decreased by 55% in C. parapsilosis (which is the second most common yeast pathogen in BSI), suggesting that it may be associated with clonal outbreaks of fluconazole-resistant C. parapsilosis. Decreased fluconazole sensitivity and increased echinocandin resistance in C. glabrata over the years can be explained by the co-resistance of C. glabrata to fluconazole and echinocandins. This is due to the increased use of echinocandin and fluconazole (42). Although all Candida spp. had high susceptibility to amphotericin B (6, 12, 17, 28, 34), C. glabrata had the highest resistance rate among Candida spp. (3, 20, 22, 28). In addition, C. krusei had reduced susceptibility to amphotericin B, and these isolates were also multi-drug resistant (MDR).
Although echinocandin resistance was as low as reported in the literature (2, 4, 20, 24), Israel et al. (19) reported increased resistance to caspofungin in C. glabrata (33.6%) and C. krusei (67%). In contrast, all C. glabrata and most C. krusei isolates were susceptible to another echinocandin derivative, micafungin. In all candidemia episodes, micafungin susceptibility showed a higher ratio compared to caspofungin, and this was associated with the use of caspofungin as the emprical echinocandin. MDR (resistance to 2 or more classes of antifungal agents) has been reported to be high in C. parapsilosis (33%) and C. glabrata (44%) by Cleveland et al. (4). In the study conducted by Mete et al. (12) in Turkey, 79% of the patients had MDR, of whom 22% were with C. parapsilosis, 20% with C. glabrata, and 1.3% with C. albicans over 10 years. Despite those statistics, in these data, 48.5% of the patients had MDR, of whom 19.1% were with C. parapsilosis, 13.5% with C. albicans, 5.3% with C. glabrata, 7.9% with C. krusei, and 1.1% with C. tropicalis over 5 years.
5.1. Conclusions
The mortality rate of invasive candidiasis remains high despite new antifungal drugs and recent advances in antifungal therapies. Early diagnosis and initiation of appropriate antifungal treatment may be delayed because of complex, relatively slow, and insensitive fungal culture. Empirical treatment with antifungal agents is associated with high costs, toxicities, and risk of antifungal resistance. Therefore, it is mandatory to determine and monitor Candida spp. and antifungal susceptibility testing for the selection of appropriate antifungal agents as empiric treatment of suspected infection.