1. Background
Escherichia coli is a Gram-negative bacterium that is commensal in the microbiota of humans and numerous animals. The ability of the bacterial genome to survive by adapting rapidly to environmental changes has caused this organism to transform into pathogenic strains that cause diseases important to public health in humans and animals. Pathogenic E. coli is split into two main groups according to the region of disease, namely intestinal pathogenic E. coli and extraintestinal pathogenic E. coli (ExPEC). The ExPEC has been implicated in numerous extraintestinal infections, such as urinary tract infections (UTIs), bloodstream infections, and pneumonia (1).
Escherichia coli is the most common reason of bloodstream infections and community-acquired sepsis. These infections are usually formed as complications of urinary and gastrointestinal tract infections (2). Phylogenetic grouping is a system used to determine the origin of the isolate and investigate the distribution of virulence factor genes. Escherichia coli is generally divided into seven different phylogenetic groups named A, B1, B2, C, D, E, and F. The ExPEC clinical isolates mostly belong to the B2 group and less frequently to D phylogenetic group. Commensal E. coli is observed more intensely in groups A and B1 (1). The ExPEC strains have multiple virulence factor genes responsible for pathogenesis, often encoded by their pathogenicity island (PAI) and other mobile deoxyribonucleic acid (DNA) elements, including papC, papG, iroN, fyuA, iutA, ompT, tsh, hlyF, hlyA, and iss (3, 4).
2. Objectives
This study aimed to investigate phylogenetic groups, various virulence factor genes, and PAI presence of E. coli isolates isolated from the fecal samples of healthy volunteers and extraintestinal isolates isolated from the blood and urine of the same patient (simultaneously).
3. Methods
3.1. Working Group
Between January 2016 and December 2017, 50 E. coli isolates were isolated from the simultaneous blood and urine samples of 25 patients hospitalized in Mersin University Faculty of Medicine, and 50 commensal E. coli isolates were isolated from the fecal 18 samples of healthy volunteers as the control group was included in the study.
3.2. Bacteria Identification
Bacteriological cultures of blood and urine samples taken from hospitalized patients were performed. Blood culture was performed with the VersaTREK (Thermo Scientific™ Remel™, Waltham, Massachusetts, USA) automated blood culture system according to the manufacturer’s recommendations. Urine cultures were inoculated on blood agar and eosin methylene blue medium as colony count and incubated at 37 °C for 18-24 hours. Then, growth was evaluated. Bacteria identification was made by classical biochemical tests. For molecular studies, the isolates were stored in broth with glycerol at -20°C (5).
3.3. DNA Extraction
For the determination of the presence of phylogenetic groups, virulence factor genes, and PAI genes, DNA isolation was performed by the phenol-chloroform method (6).
3.4. Multiplex Polymerase Chain Reaction
The presence of phylogenetic groups, virulence factor genes, and PAI genes was determined by multiplex polymerase chain reaction (PCR) using the same amplification conditions. The PCR amplification of each sample was performed in a reaction volume of 50 µL. The PCR reaction mix contains 5 μL of 10 x PCR buffer, 4 μmol/μL magnesium chloride, 1 μmol/μL deoxyribonucleotide triphosphate mix, 0.25 pmol/μL each primer, 0.25 μL Taq DNA polymerase, and 5 μL sample DNA. The amplification conditions of the samples included the initial denaturation at 94°C for 5 minutes, followed by 30 cycles of 30 seconds of denaturation at 94°C, 30 seconds of annealing at 55°C, and 30 seconds of extension steps at 72°C. Finally, 72°C was applied as the last elongation step for 7 minutes (6). Amplification products were visualized under ultraviolet light after electrophoresis at 130 volts for 40 minutes on a 1% agarose gel containing 0.5 μg/mL ethidium bromide. The size of the amplicons was assessed with a 1000 bp DNA molecular weight marker (GeneRuler 100 bp Plus DNA Ladder, Thermo Scientific™, Waltham, Massachusetts, USA).
3.5. Phylogenetic Classification
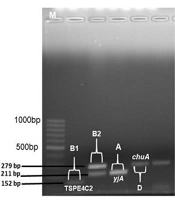
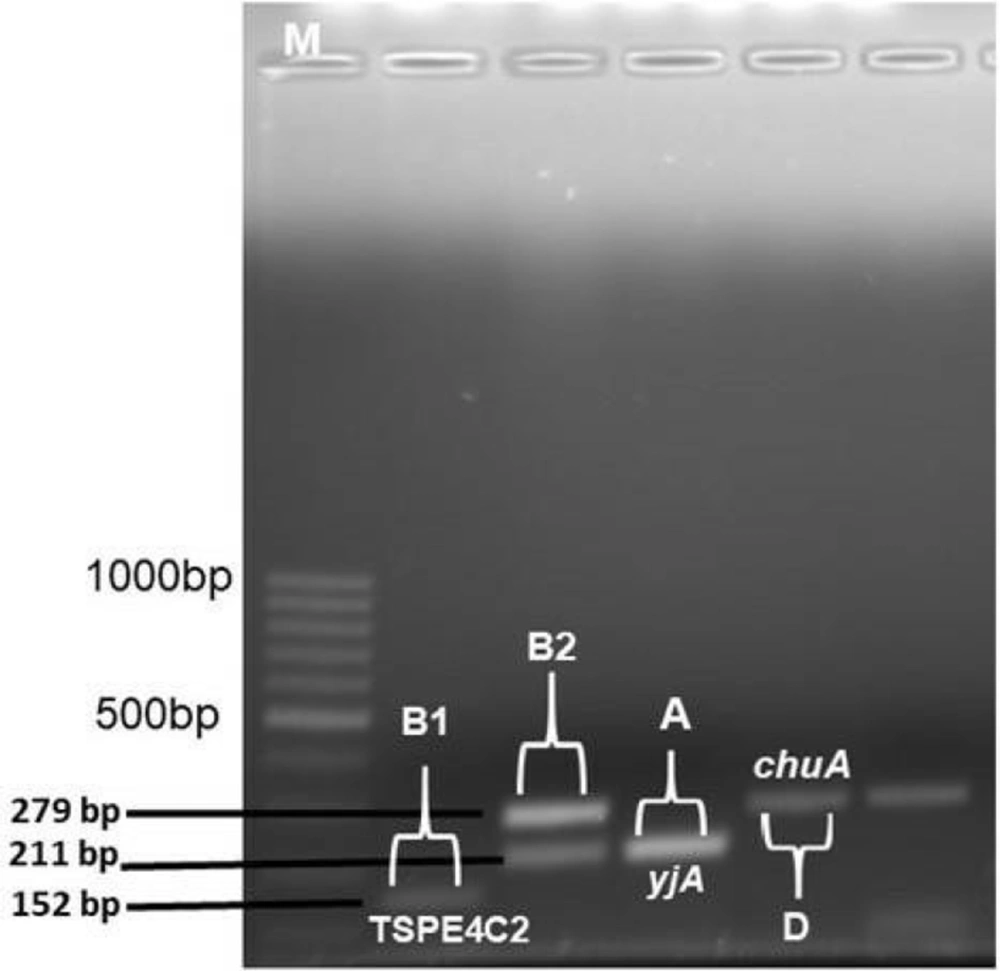
Phylogenetic groups A, B1, B2, and D were determined by multiplex PCR. In grouping, chuA and yjaA genes and DNA fragment TSPE4.C2 were used. Primer pair sequences designed by Clermont et al. were used for PCR detection of chuA and yjaA genes and TSPE4.C2 DNA fragment (6).
3.6. Detection of Virulence Factor Genes
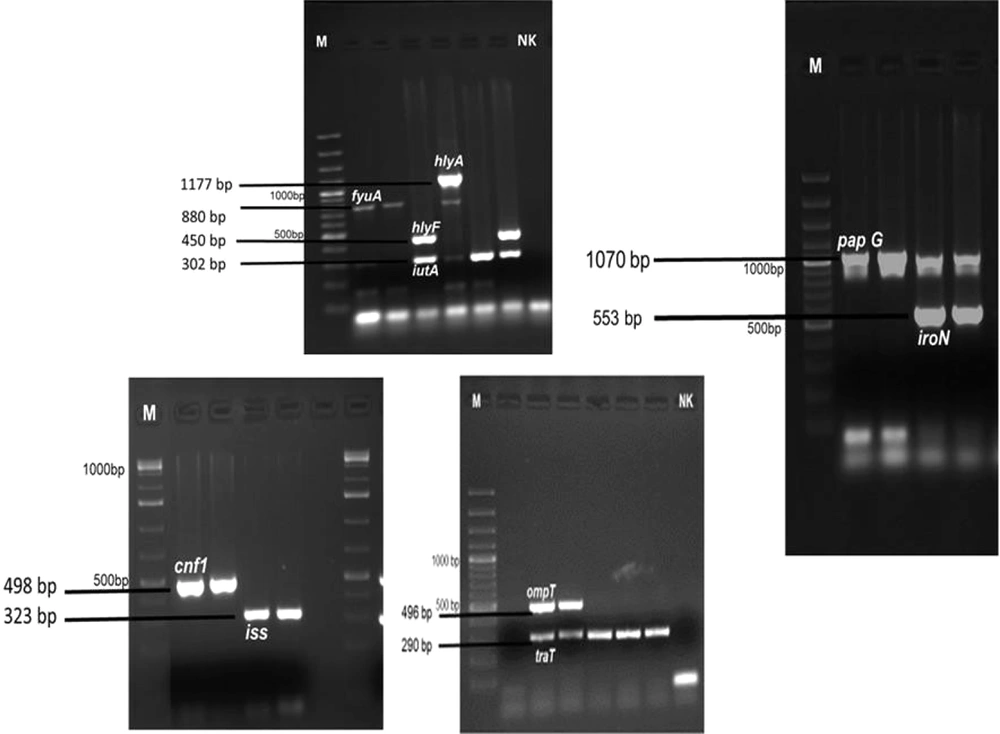
Virulence factor genes (i.e., pap C, pap G, iutA, fyuA, iroN, traT, iss, hlyF, cnf1, hlyA, and ompT) were determined by the multiplex PCR method. Primer sequences designed by Johnson et al. were used to identify virulence factor genes (7, 8).
3.7. Detection of Pathogenicity Islands
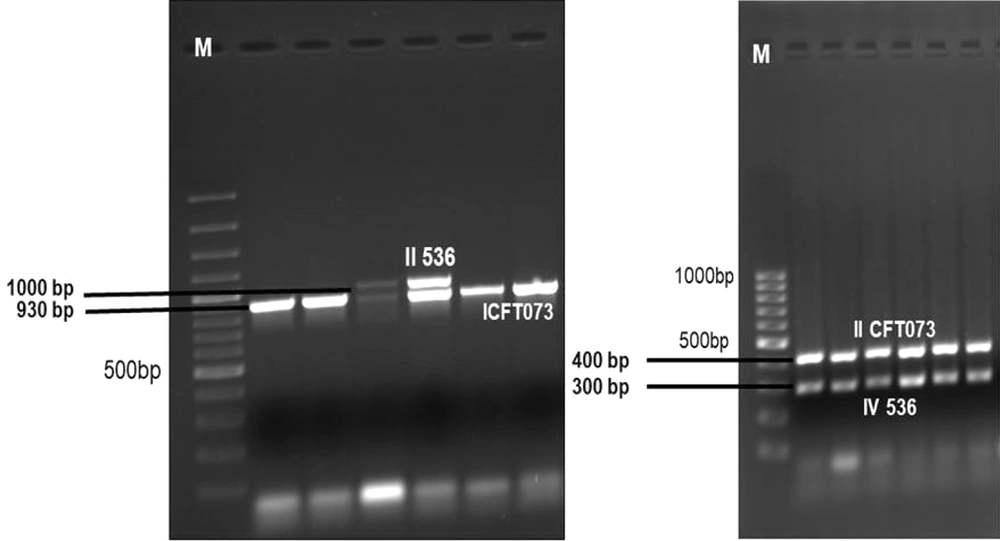
Seven PAI markers (i.e., ICFT073, IICFT073, I536, II536, IV536, IJ96, and IIJ96) were determined by the multiplex PCR method. The primer sequences designed by Sabate et al. were used to determine PAIs (4).
3.8. Statistical Methods
The statistical analysis of the data was made with Statistica software (version 13.5.0.17). The Chi-square homogeneity test was applied to determine the relationship of virulence factor genes with the phylogenetic group. Statistical significance level ≤ 0.05 was accepted.
4. Results
4.1. Phylogenetic Group Distribution
Of the ExPEC isolates, 22 (44%), 13 (26%), 11 (22%), and 4 (8%) isolates were detected in groups B2, D, A, and B1, respectively (Table 1). All 50 commensal E. coli isolates were observed to be in phylogenetic group A. Figure 1 depicts phylogenetic group gene regions of E. coli isolates.
| Phylogenetic Group | Total (Blood and Urine) (n = 50) | Fecal (n = 50) |
|---|---|---|
| A | 11 (22) | 50 (100) |
| B1 | 4 (8) | - |
| B2 | 22 (44) | - |
| D | 13 (26) | - |
| Virulence factor genes | ||
| Adhesins | ||
| pap C (P fimbriae) | 5 (10) | - |
| papG (P fimbriae adhesin) | 28 (56) | - |
| Toxins | ||
| hlyF (hemolysin F) | 11 (22) | 25 (50) |
| hlyA (α-hemolysin) | 1 (2) | - |
| cnf1 (cytotoxic necrotizing factors) | 7 (14) | - |
| Siderophore | ||
| iut A (aerobactin siderophore receptor) | 35 (70) | 40 (80) |
| fyuA (yersiniabactin siderophore receptor) | 6 (12) | - |
| iroN (salmochelin siderophore receptor) | 12 (24) | 10 (20) |
| Serum resistance | ||
| traT | 38 (76) | 35 (70) |
| iss (serum resistance-associated protein) | 6 (12) | 15 (30) |
| ompT (episomal outer membrane protease) | 10 (20) | 20 (40) |
| Pathogenicity island | ||
| ICFT073 | 20 (40) | - |
| IICFT073 | 22 (44) | - |
| II 536 | 4 (8) | - |
| IV 536 | 43 (86) | 20 (40) |
Distribution of Virulence Factor Genes, Pathogenicity Islands, and Phylogenetic Groups in Escherichia coli Strains Isolated from Clinical Specimens
4.2. Virulence Factor Genes
This study examined the distribution of 11 virulence factor genes encoding 10 virulence factor genes. One or more virulence factor genes were detected in 47 (94%) of 50 ExPEC isolates, and virulence factor genes’ regions could not be detected in 3 isolates. The most common traT (serum resistance) gene (n = 38, 76%) and iutA (aerobactin) gene (n = 35, 70%) were detected in ExPEC isolates. Other virulence factor gene distribution in Expec isolates was also reported as 56% papG, 24% iroN, 22% hlyF, 20% ompT, 14% cnf1 (n = 7), 12% iss, 12% fyuA, 10% papC, and 2% hlyA. The most common virulence factor genes region of iutA (80%) and traT (70%) were detected in commensal E. coli isolates. Figure 2 illustrates the virulence factor genes’ regions of E. coli isolates. Table 1 shows the distribution of phylogenetic groups, PAI, and virulence factor gene regions of E. coli isolates.
4.3. Pathogenicity Islands
A total of 86 PAIs were detected in 45 (90%) of the 50 ExPEC isolates. No PAIs were detected in five isolates. The most common PAI was determined to be IV536 (n = 43, 86%). The IICFT073 (44%) of the isolates, ICFT073 (40%), and to a lesser extent II536 (8%) PAI were detected in 20 of the isolates. The PAI I536, IJ96, and IIJ96 markers were not detected in any isolate. The PAIs were detected in only 20 (40%) commensal E. coli isolates, and only PAI IV 536 was detected. Figure 3 depicts the PAI gene regions of E. coli isolates. Table 1 shows the virulence factor, PAI, and phylogenetic group distribution detected in commensal and ExPEC isolates.
4.4. Relationship Between Phylogenetic Groups and Virulence Factor Genes
The most common traT, iutA, and papG virulence factor genes in ExPEC isolates were mostly detected in the B2 phylogenetic group; however, hlyF, ompT, and iss virulence factor genes were most frequently detected in group A, and papC was most frequently detected in group D isolates.
4.5. Relationship Between Pathogenicity Islands and Phylogenetic Groups
The relationship between PAIs and phylogenetic groups was statistically significant (P ≤ 0.05). It was determined that 69.7% of PAIs were detected in ExPEC isolates belonging to group B2. PAI IICFT073 was detected in 81.8%, 50%, and 18.2% of isolates in B2, B1, and A groups. PAI IV536 was detected in 100%, 90.9%, 76.9% and 50% of isolates in B2, A, D and B1 groups, respectively. It was observed that the detection of high IV536 and IICFT073 PAI rates in ExPEC isolates in Group B2 was statistically significant (P ≤ 0.05).
5. Discussion
The ExPEC can colonize the urinary system with various virulence factors and might escape from the host defense and cause serious infections, such as bacteremia and sepsis. The ExPEC strains have genes encoding virulence factors, such as adhesins, toxins, invasins, iron recovery systems, and encapsulation. The ExPEC strains contain more virulence factors than commensal E. coli strains. These strains cause attachment to the host cell with virulence factors, survival in the host, and damage to tissues and cells (2). In this study, it was observed that the serum resistance factor genes traT, iss, and ompT were detected at the same rates in blood, urine, and stool isolates. Consistent with other studies, serum resistance factor (traT) was detected with a high frequency in isolates, causing sepsis (1, 8-13). Commensal originated E. coli isolates might gain additional virulence factors and cause UTI and sepsis.
Iron uptake systems include aerobactin and yersinia baktin siderophores, which are necessary for bacteremia and for an invasive infection to occur (14). According to this study, IutA (70%) virulence factor gene that has the second frequency was detected in ExPEC isolates. The rate of this gene region was reported as 90.9% by Malekzadegan et al. (14), and Paniagua-Contreras et al. (15) reported it as a low rate (16.5%). Daga et al. observed fyuA (70.8%) most frequently in bloodstream infection isolates, followed by iutA (64.3%) and iroN (37.5%), respectively (1). In this study, iutA (76%), iroN (28%), and fyuA (12%) gene regions were observed most frequently in bloodstream infection isolates. Commensal isolates with the same characteristics as ExPEC can be isolated under pathogenic conditions, thereby revealing the role of host-dependent factors in the development of infection (16).
According to numerous meta-analysis studies, it has been shown that the most frequently encoded toxin genes in ExPEC are tsh, hlyA, hlyF, and cnf1. In this study, the rate of the hlyA gene in urinary isolates (2%) was observed to be lower than the results of other studies on this subject (14, 17, 18). Daga et al. observed the hlyA gene at a rate of 14.6% in bloodstream infection isolates (1). In the current study, the hlyA gene was not detected in isolates isolated from the blood samples. In commensal isolates, the hlyF gene was observed at a rate of 50%, and hlyA and cnf1 were not detected. In the studies, there was a significant relationship between the hly gene and ExPEC isolates isolated from urosepsis, and the low rate obtained in this study is thought to be related to the clinical characteristics of the patients (17).
Numerous surfaces of the structures play an important role in the specific adhesion process. S fimbrial adhesins (sfa) and P-like pili (e.g., papC, papG, and iha) are the most commonly detected adhesins among isolates isolated from UTI patients (12). According to a study by Qin et al., the prevalence of P-type fimbrial adhesive genes was reported in 28% of ExPEC isolates and 5% of commensal strains (19). In a study by Shetty et al., it was reported that 30.4% of isolates with adhesive-encoding genes had two genes, pap, and sfa (20). In this study, it was determined that ExPEC isolates carry 56.6% of the papG gene and 10% of the papC gene; nevertheless, these genes were not detected in commensal strains. According to the evidence, the frequency of the pap gene has been reported to vary within 25 - 77% (3, 8, 17, 18). Strains without the Pap operon can use other adhesins for binding to urinary epithelial cells and initiate infection.
The PAIs and their associated virulence factor genes were spread through bacteria populations by horizontal transmission (21). Samei et al. detected PAI markers in a significant proportion of commensal (88%) and uropathogenic E. coli (UPEC) (98.6%) isolates and reported that PAI IV53 (98.7% UPEC and 84% commensal) was the most common in the two groups (21). Li et al. reported that commensal E. coli isolates carried PAI at a rate of 46.8%, with PAI IV536 (38.2%) and PAI ICFT073 (20.9%) as the most common (22). Sebat et al. detected PAI in 40% of commensal isolates and 93% of UPEC, with PAI IV536 (38% commensal, 89%UPEC) and PAI ICFT073 as the most common (4). Navidinia et al. reported that 89% of UPEC isolates contained PAI, frequently PAI IV536 (86%) and PAI ICFT073 (74%) (23). In this study, PAI was detected in 90% of ExPEC isolates and 40% of commensal isolates. The most common PAI was IV536 (100% commensal and 95.5% ExPEC). It was reported that PAI is common among commensal and pathogenic strains, and commensal isolates might be reservoirs for the transmission of these markers (21).
The ExPEC and commensal E. coli isolates can be clustered in different phylogenetic groups. In this study, it was determined that group B2 (44%) was the most common among ExPEC isolates, followed by group D (26%), group A (22%), and group B1 (8%), and all commensal isolates were detected in group A. Koga et al. observed the most common group B2 (40%) in blood culture isolates and group A (72.54%) the most common in commensal isolates (10). Bozcal et al. reported the most common group as D (38.14%) in blood culture isolates, followed by A (29.89%), B2 (20.61%), and B1 (11.34%) (11). Sannes et al. showed that bacteremia and rectal isolates were the most common in the B2 group; however, this rate was higher in bacteremia isolates (24). Martinez et al. reported that ExPEC isolates causing bloodstream infection are mostly in the D phylogenetic group (25). Duriez et al. reported that commensal E. coli strains obtained from three geographically different regions (i.e., France, Croatia, and Mali) were most commonly in phylogenetic groups A (40%) and B1 (34%) (26).
When the relationship between virulence genes and phylogenetic groups was examined in this study, a statistically significant relationship was observed between the B2 phylogenetic group and virulence factor genes (P < 0.05). The most common virulence factor genes traT, iutA, and papG were detected in ExPEC isolates belonging to the B2 phylogenetic group. The most common traT and iutA virulence factor genes were detected in commensal E. coli isolates in the A phylogenetic group. Bozcal et al. determined the most fyuA virulence factor gene region in commensal E. coli isolates in the A phylogenetic group (11).
According to a study by Cyoia et al., it was reported that the fyuA virulence factor gene region was detected in ExPEC strains, and PAIs were mostly detected in the B2 phylogenetic group (27). As a result, it has been determined that each isolate might be mediated by one or more virulence factor genes in the uropathogenic and bloodstream infection process, and each isolate might have a unique combination of these factors. This finding indicates that ExPEC isolates have a higher frequency of PAIs. The PAI IV536 was most frequently detected in commensal and ExPEC isolates. This result indicates that commensal isolates might be reservoirs for the transfer of PAI markers. Additionally, 44% of ExPEC isolates were detected in the B2 phylogenetic group. Although the B2 phylogenetic group corresponds to the virulence potential, the results of the present study showed that isolates in groups A (commensal) and D might also cause extraintestinal infections.
5.1. Conclusions
It was concluded that host factors are also important in the development of infection, as well as the presence of virulence factors and PAIs. There are numerous studies on this subject worldwide; however, a limited number of studies have been performed in Turkey. This was a comprehensive study investigating the virulence factors of ExPEC isolates in Turkey, and the authors believe that it will contribute to studies on this subject.