1. Background
To date, human Coronaviruses have led to three epidemics, including severe acute respiratory syndrome Coronavirus (SARS-CoV), middle east respiratory syndrome Coronavirus (MERS-CoV), and severe acute respiratory syndrome Coronavirus 2 (SARS‐CoV‐2). The emerging Coronavirus disease 2019 (COVID‐19) caused by SARS‐CoV‐2 was first reported in Wuhan, China, in 2019. This pandemic has marked one of the century's catastrophes (1). Coronavirus disease 2019 is clinically diverse and widespread so that most people with COVID-19 often have mild and no severe symptoms of the disease while a few experience severe COVID‐19, requiring care, oxygen support, and hospitalization. Moreover, many patients are admitted to intensive care units (2). Manifestations may be asymptomatic. Mild symptoms such as fever and cough or more severe symptoms such as acute respiratory distress syndrome (ARDS) may also occur, eventually leading to death (3). Following SARS-CoV-2, lymphopenia occurs, resulting in a cytokine storm. It also causes pathological problems in the lungs, liver, heart, and other body organs (4).
Worldwide, approximately 24 million people have been identified with SARS-CoV-2. Older people with risk factors and chronic diseases, including obesity, respiratory disease, diabetes, cardiovascular disease (CVD), and high blood pressure (hypertension), have more severe symptoms and higher mortality and morbidity rates. Further, many HIV-infected people are at risk of COVID-19 (5). Little is known about the HIV impact on COVID-19. Individuals infected with HIV-1 are usually immunocompromised, and it is unclear how a suppressed immune system leads to SARS-CoV-2 (6). According to the World Health Organization (WHO) latest statistics, it was estimated that by the end of 2019, about 38.0 million people would be infected with HIV (7). Besides, HIV-1-infected people, particularly those with low CD4 cell counts, possibly are at an increased risk for severe diseases or COVID-19, and the HIV viral load is not suppressed in these people (8). The regular consumption of antiretrovirals (ARVs), including nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs) and immunosuppressors, might change clinical manifestations and risk of COVID-19 in HIV-1-infected individuals (9).
Previous studies of MERS-CoV and SARS-CoV have mostly reported a lower risk of severe disease in HIV-infected patients. These studies report the inhibition of Coronavirus replication by ARVs to reduce Coronavirus risk in HIV-infected individuals. However, due to the suppression of the immune system, the disease lasts longer in these patients (10). Probably, the risk of COVID-19 increases in HIV-infected individuals with a high HIV viral load and low CD4 cell count (8). On the other hand, protection against cytokine storms due to SARS-CoV-2 has been reported by reducing the number of CD4 cell counts and suppressing the immune system in HIV-1-infected individuals (11). However, subsequent research has reported that COVID-19 severity is associated with low CD4 cell counts (12).
Several researchers believe that high sensitivity to SARS-CoV-2 results from the suppression of the immune system by HIV, while others believe that these people have less risk and complications due to problems associated with cellular immunity. Besides, HIV-1-infected individuals probably have lower inflammation, reducing COVID-19-induced cytokine storm (13). Moreover, researchers have different opinions about the effectiveness of ARVs against COVID-19 (14). Although there is no exact information about the risk of infection with SARS-CoV-2 in HIV-1-infected individuals, these individuals are probably at greater risk of COVID-19 (15). After the COVID-19 pandemic, about 19% of HIV-1-infected individuals reportedly did not have access to ARVs (16). In HIV-infected patients, the use of ARVs has increased life expectancy, but the COVID-19 pandemic, along with HIV, has posed significant challenges in managing and controlling this group of patients (17). So far, not much research has reported the epidemiological features and clinical outcomes of patients with HIV/SARS-CoV-2 confection. Following the COVID-19 pandemic, concerns have been raised about the lack of data, particularly in countries with high HIV rates, including Sub-Saharan Africa, which accounts for 70% of HIV infections (18).
2. Objectives
This study evaluated the SARS-CoV-2 infection rate and COVID-19 prevalence among Iranian HIV-1-infected patients.
3. Methods
3.1. Patients Selection
From June 2020 to October 2020, 155 consecutive Iranian HIV-1-infected patients were examined in this cross-sectional study. These patients were treated in clinics or hospitals affiliated with the Iran University of Medical Sciences (IUMS), Tehran, Iran. The treatment regimen of these 155 individuals was as follows: (1) NRTIs and NNRTIs-based regimens in 110 (70.5%); (2) NRTIs and PIs-based regimens in 10 (6.4%); (3) NRTIs, PIs, and INIs-based regimens in two (1.3%); (4) NRTIs, NNRTIs, and PIs-based regimens in four (2.6%); and (5) NRTIs, NNRTIs, PIs, and INIs-based regimens in two (1.3%). One (1.3%) patient was not taking any antiretroviral drugs.
3.2. Sample Collection
Nasopharyngeal and oropharyngeal samples were taken from HIV-1-infected patients, placed in the virus transport medium (VTM), and sent to the laboratory to diagnose infection with the COVID-19 virus. To detect antibodies against the virus, 3 mL of the peripheral blood was taken from the participants, and the plasma of the samples was separated by centrifugation and then frozen at -80°C until use.
3.3. Detection of COVID-19 Ab (IgG) in Serum Using Enzyme Immunoassay (EIA)
COVID-19 Ab (IgG) in serum samples was analyzed using an enzyme immunoassay (EIA) kit (Euroimmun Medizinische Labordiagnostika, Lubeck, Germany) according to the manufacturer's instructions.
3.4. Viral RNA Extraction and cDNA Synthesis
Viral RNA was isolated from the nasopharyngeal and oropharyngeal samples using a High Pure Viral Nucleic Acid (Roche Diagnostics GmbH, Mannheim, Germany) kit, according to the manufacturer’s instructions. The quality and quantity of the extracted viral RNA were determined by a nanodrop spectrophotometer (Thermo Scientific, Wilmington, MA) and kept at -20°C. The cDNA synthesis was carried out at 42°C for 30 min in a 20 µL reaction mixture containing random hexamer (20 pmol), 5 X reverse transcriptase (RT) reaction buffer (4 µL), 102 U of Moloney murine leukemia virus (MMuLV) reverse transcriptase (Fermentas GmbH, St. LeonRot, Germany), 18.4 U of RNase inhibitor (Fermentas GmbH, St. Leon-Rot, Germany), dNTPs (100 mmol), 1.0 µL of DEPC-treated water, and 0.5 mg of the isolated RNA. The reverse transcriptase enzyme was inactivated after cDNA synthesis at 72°C for 10 min.
3.5. SARS-CoV-2 Detection by Real-Time Polymerase Chain Reaction
The envelope (E) and RdRp (RNA-dependent RNA polymerase) regions of SARS-CoV-2 in the extracted RNA were detected using real-time polymerase chain reaction (RT-PCR) with specific primers and TaqMan probes for these regions (E, RdRp genes) (19), using RNase P as an internal control (20) (Table 1). The Rotor-Gene Q (QIAGEN, Germany) instrument was used for the experiment. For positive and negative controls, the nasopharyngeal and oropharyngeal specimens of five patients with SARS-CoV-2 infection and five healthy people were used, respectively. Briefly, the real-time PCR was carried out using 25 μL reaction mixtures containing 12.5 μL Premix Ex TaqTM (probe qPCR) (TaKaRa Bio Inc. Shiga, Japan), 10 pmol each specific primer and 5 pmol each TaqMan probe for E, RdRp genes, with RNase P and 5 μL of the cDNA as a template. The reaction was carried out at 95°C for 10 min and 40 cycles of amplification (95°C for 10 s and 60°C for 40 s).
| Assay Use and Polarity | Names | Sequences |
|---|---|---|
| E | ||
| Forward primer | E-Sarbeco | ACA GGT ACG TTA ATA GTT AAT AGC GT |
| Reverse primer | E-Sarbeco | ATA TTG CAG CAG TAC GCA CAC A |
| Probe | E-Sarbeco | FAM- ACA CTA GCC ATC CTT ACT GCG CTT CG -BBQ |
| RdRp | ||
| Forward primer | RdRP-SARSr | GTG ARA TGG TCA TGT GTG GCG G |
| Reverse primer | RdRP-SARSr | CAR ATG TTA AAS ACA CTA TTA GCA TA |
| Probe | RdRP-SARSr | VIC- CAG GTG GAA CCT CAT CAG GAG ATG C -BBQ |
| RNase p | ||
| Forward primer | RP2-F | AGA TTT GGA CCT GCG AGC G |
| Reverse primer | RP2-R | GAG CGG CTG TCT CCA CAA GT |
| Probe | RP2-probe | ROX- TTC TGA CCT GAA GGC TCT GCG CG -BBQ |
Abbreviations: E, envelope; RdRp, RNA-dependent RNA polymerase; RNase P, ribonuclease P.
3.6. Statistical Analysis
Statistical analysis was conducted by SPSS version 20 software (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to assess the normality of the data. The chi-square test or Fisher’s exact test determined the statistical differences between categorical variables. A P value of less than 0.05 was considered statistically significant.
4. Results
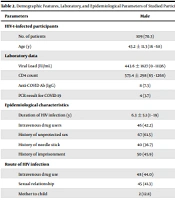
All 155 HIV-1-infected patients (anti-HIV Abs and HIV-RNA positive) referred to the clinics and hospitals affiliated with IUMS, Tehran, Iran, were enrolled in the present research. The mean age of the patients was 43.2 ± 11.3 years (range 16 - 68 years). Of the 155 participants, 109 (70.3%) were males. Demographic and epidemiological characteristics and laboratory data are presented in Table 2. The genomic RNA of SARS-CoV-2 was detected in the nasopharyngeal and oropharyngeal samples of 12 (7.7%) HIV-infected individuals, of whom four (33.3%) were males. It is noteworthy that 10 (6.5%) subjects were positive for antibodies against the virus, so they had already been infected with SARS-CoV-2 and had antibodies against it (Table 2).
| Parameters | Male | Female | Total |
|---|---|---|---|
| HIV-1-infected participants | |||
| No. of patients | 109 (70.3) | 46 (29.7) | 155 (100) |
| Age (y) | 43.2 ± 11.3 (16 - 68) | 43.0 ± 11.5 (26 - 68) | 43.2 ± 11.3 (16 - 68) |
| Laboratory data | |||
| Viral Load (IU/mL) | 443.6 ± 1827 (0 - 11136) | 30.2 ± 68 (0 - 214) | 320.9 ± 1542 (0 - 11136) |
| CD4 count | 573.4 ± 298 (65 - 1268) | 679.2 ± 305 (67 - 1481) | 604.8 ± 303 (65 - 1481) |
| Anti-COVID Ab (IgG) | 8 (7.3) | 2 (4.3) | 10 (6.5) |
| PCR result for COVID-19 | 4 (3.7) | 8 (17.4) | 12 (7.7) |
| Epidemiological characteristics | |||
| Duration of HIV infection (y) | 6.3 ± 5.1 (1 - 19) | 5.7 ± 5.3 (1 - 19) | 6.1 ± 5.2 (1 - 19) |
| Intravenous drug users | 46 (42.2) | 14 (30.4) | 60 (38.7) |
| History of unprotected sex | 67 (61.5) | 18 (39.1) | 85 (54.8) |
| History of needle stick | 40 (36.7) | 0 (0.0) | 40 (25.8) |
| History of imprisonment | 50 (45.9) | 0 (0.0) | 50 (32.3) |
| Route of HIV infection | |||
| Intravenous drug use | 48 (44.0) | 0 (0.0) | 48 (31.0) |
| Sexual relationship | 45 (41.3) | 38 (82.8) | 83 (53.5) |
| Mother to child | 2 (12.8) | 0 (0.0) | 2 (1.3) |
| Unknown | 14 (12.8) | 8 (17.4) | 22 (14.2) |
| Marital status | |||
| Single | 43 (39.4) | 5 (10.9) | 48 (31.0) |
| Married | 45 (11.1) | 24 (52.2) | 69 (44.5) |
| Divorced | 14 (12.8) | 11 (23.9) | 25 (16.1) |
| Widow | 7 (6.4) | 6 (13.0) | 13 (8.4) |
| Education | |||
| Under diploma | 50 (45.9) | 14 (30.4) | 64 (41.3) |
| Diploma | 31 (28.4) | 18 (39.1) | 49 (31.6) |
| Upper diploma | 26 (23.9) | 14 (30.4) | 40 (25.8) |
| Unknown | 2 (1.8) | 0 (0.0) | 2 (1.3) |
a Values are expressed as No. (%) or mean ± SD (range).
The mean viral load of the studied patients was 320.9 ± 1542 IU/mL (range 0 - 11136). The mean CD4 count of the patients was 604.8 ± 303 cells/µL (range 65 - 1481). The mean duration of HIV infection was 6.1 ± 5.2 years (range 1 - 19). Injecting drug use (IDU), history of unprotected sex, history of needle sticks, and history of imprisonment were positive in 60 (38.7%), 85 (54.8%), 40 (25.8%), and 50 (32.3%) patients, respectively. Also, concerning the routes of HIV infection, IDU, sexual relationship, mother to child transmission, and unknown routes were reported in 48 (31.0%), 83 (53.5%), two (1.3%), and 22 (14.2%) patients, respectively. Anti-COVID Ab (IgG) was detected in 10 (6.5%). However, the real-time PCR result for SARS-CoV-2 infection was positive in 12 (7.7%) (Table 2).
Clinical symptoms of the studied HIV-1-infected participants with positive and negative results of real-time PCR for SARS-CoV-2 infection were evaluated for various symptoms such as fever, confusion, headache, chills, and runny nose, among others. In HIV-1-infected patients with SARS-CoV-2, the most common clinical symptoms were dry cough in seven (58.3%), fever in five (41.7%), runny nose in five (41.7%), smell dysfunction in five (41.7%), and taste dysfunction in five (41.7%). Furthermore, HCV Ab was detected in four (33.3%) of the HIV-infected individuals diagnosed with COVID-19. In addition, 10 (83.3%) of these patients showed HBsAb. However, none of the HIV-1-infected individuals with SARS-CoV-2 infection were detected with HBsAg, diabetes, tuberculosis (TB), and Kaposi's sarcoma (Table 3).
| Parameters | Real-Time PCR/Positive | Real-Time PCR/Negative |
|---|---|---|
| Sex | ||
| Male | 4 (33.3) | 105 (73.4) |
| Female | 8 (66.7) | 38 (26.6) |
| Symptoms | ||
| Fever | 5 (41.7) | 4 (2.8) |
| Confusion | 0 (0.0) | 2 (1.4) |
| Headache | 3 (25.0) | 10 (7.0) |
| Chills | 2 (16.7) | 2 (1.4) |
| Skeletal pain | 3 (25.0) | 11 (7.7) |
| Dry cough | 7 (58.3) | 12 (8.4) |
| Sputum cough | 0 (0.0) | 6 (4.2) |
| Chest pain | 0 (0.0) | 0 (0.0) |
| Shortness of breath | 0 (0.0) | 0 (0.0) |
| Runny nose | 5 (41.7) | 8 (5.6) |
| Cape of nose | 0 (0.0) | 6 (4.2) |
| Deceased smell | 5 (41.7) | 4 (2.8) |
| Deceased taste | 5 (41.7) | 4 (2.8) |
| Gastrointestinal symptom | 2 (16.7) | 3 (2.1) |
| Bleeding stomach | 0 (0.0) | 0 (0.0) |
| Other | ||
| HCV Ab | 4 (33.3) | 27 (18.9) |
| HBsAg | 0 (0.0) | 2 (1.4) |
| HBsAb | 10 (83.3) | 115 (80.4) |
| Diabetes | 0 (0.0) | 2 (1.4) |
| Tuberculosis (TB) | 0 (0.0) | 4 (2.8) |
| Kaposi's sarcoma | 0 (0.0) | 2 (1.4) |
a Values are expressed as No. (%).
In this study, the participants had different types of blood groups. Out of 12 (7.7%) positive real-time PCR results for SARS-CoV-2, the numbers of HIV-1-infected patients with the O+ blood group, A+ blood group, B+ blood group, and AB+ blood group were four, three, three, and two, respectively. Out of 10 (6.5%) positive results for anti-COVID Ab (IgG), the numbers of HIV-1-infected patients with the O+ blood group, O- blood group, and A+ blood group were six, two, and two, respectively (Table 4).
| No. | Sex/Age | Blood Type | COVID-19 Ab | Real-Time PCR | Duration of HIV Infection (y) | CD4 Count | Viral Load (IU/mL) | Antiretroviral Drugs Used by Patients | HIV-1 Subtype |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/57 | O+ | + | + | 14 | 176 | 0 | NRTIs, NNRTIs | CRF35_AD |
| 2 | M/30 | O- | + | - | 3 | 872 | 182 | NRTIs, NNRTIs | CRF35_AD |
| 3 | M/33 | O+ | + | - | 15 | 860 | 0 | NRTIs, INIs | CRF35_AD |
| 4 | F/41 | A+ | + | - | 1 | 902 | 0 | NRTIs, NNRTIs | CRF35_AD |
| 5 | M/50 | O+ | + | - | 11 | 429 | 2275 | NRTIs, NNRTIs, PIs | CRF01_AE |
| 6 | M/47 | B+ | - | + | 3 | 610 | 0 | NRTIs, NNRTIs | CRF35_AD |
| 7 | F/58 | O+ | - | + | 12 | 884 | 0 | NRTIs, NNRTIs | CRF35_AD |
| 8 | F/26 | A+ | - | + | 2 | 337 | 214 | NRTIs, INIs | CRF35_AD |
| 9 | F/32 | B+ | - | + | 4 | 515 | 0 | NRTIs, NNRTIs | CRF35_AD |
| 10 | F/28 | AB+ | - | + | 1 | 1001 | 0 | NRTIs, NNRTIs | CRF35_AD |
| 11 | M/31 | O+ | + | - | 13 | 803 | 0 | NRTIs, INIs | CRF35_AD |
| 12 | M/52 | O+ | + | - | 9 | 399 | 2040 | NRTIs, NNRTIs, PIs | CRF35_AD |
| 13 | F/26 | AB+ | - | + | 1 | 998 | 0 | NRTIs, NNRTIs | CRF35_AD |
| 14 | M/45 | A+ | - | + | 4 | 643 | 0 | NRTIs, NNRTIs | CRF35_AD |
| 15 | F/30 | B+ | - | + | 2 | 537 | 0 | NRTIs, NNRTIs | CRF35_AD |
| 16 | F/55 | O+ | - | + | 11 | 829 | 0 | NRTIs, NNRTIs | CRF35_AD |
| 17 | M/31 | O- | + | - | 4 | 853 | 185 | NRTIs, NNRTIs | CRF35_AD |
| 18 | F/28 | A+ | - | + | 3 | 402 | 221 | NRTIs, INIs | CRF35_AD |
| 19 | F/39 | A+ | + | - | 1 | 898 | 0 | NRTIs, NNRTIs | CRF35_AD |
| 20 | M/56 | O+ | + | + | 12 | 189 | 0 | NRTIs, NNRTIs | CRF35_AD |
Abbreviations: NRTIs, nucleoside reverse transcriptase inhibitors; NNRTIs, non-nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors; INIs, integrase inhibitors.
5. Discussion
It is currently known that most individuals, including pregnant women and children, are at risk of infection with SARS-CoV-2. Several studies have shown that older individuals are more susceptible to COVID-19 and usually have symptoms such as cough, fever, headache, and fatigue (21, 22). To our knowledge, this study is the first study on COVID-19 diagnosis in Iranian people with HIV infection. In this study, the COVID-19 infection rate in HIV-infected individuals was observed to be 7.7% (n = 12). The COVID-19 diagnosis rate was higher in females (n = 8, 17.4%) than males (n = 4, 3.7%). The participants were evaluated for various symptoms such as fever, confusion, headache, chills, and runny nose. In patients with SARS-CoV-2, the most common symptoms were dry cough (n = 7, 58.3%), fever (n = 5, 41.7%), runny nose (n = 5, 41.7%), anosmia (n = 5, 41.7%), and hypogeusia (n = 5, 41.7%). Our data demonstrated that HCV Ab was detected in four (33.3%), and HBsAb was detected in 10 (83.3%) HIV-infected patients with COVID-19. In the present study, none of the patients with the positive result of real-time PCR for SARS-CoV-2 was detected with HBsAg, diabetes, tuberculosis (TB), and Kaposi's sarcoma.
According to previous studies, a low rate of CD4 cell counts and suppressed immune systems may have a protective effect on HIV-infected patients against the cytokine storm created in individuals with COVID-19 (11). However, factors related to COVID-19 severity, including high levels of interleukin-6 and low rate of platelet counts or lymphocytes, are associated with a low rate of CD4 cell counts (23). Since low CD4 counts do not relate to COVID-19, the disease severity is likely to be affected by immunosuppression and appears to be associated with SARS-CoV-2 persistence and detrimental outcomes (12). Findings have demonstrated that COVID-19 in HIV-infected individuals due to immunosuppression can delay the SARS-CoV-2 clearance. However, the clinical recovery of COVID-19 was better in HIV-infected patients than in non-HIV-infected individuals (18, 24). Moreover, some studies demonstrated that the HIV viral load affected antibody levels against SARS-CoV-2. Infection with HIV is likely to influence the immune system's response to SARS-CoV-2, leading to harmful outcomes and permanence of SARS-CoV-2. It has recently been shown that the risk of severe COVID-19 manifestations is higher in people infected with HIV for a long time (25). Research has indicated that approximately 14% of individuals infected with SARS-CoV-2 have experienced severe illness, and about 6% have serious conditions (26).
Several studies have reported that decreased immune system potency is associated with aging (27). In previous studies, old age was a leading cause of death in MERS and SARS (28, 29). Moreover, studies have shown that women are less likely to be infected with SARS-CoV and MERS-CoV than men (30, 31). No agreement has been reached on using ARVs to prevent or treat COVID-19 (32). In HIV-1-infected individuals, the outcome and clinical stages of COVID-19 are not yet known. Several researchers have reported that HIV-1-infected individuals on ARV treatment may experience a lower risk of COVID-19 and relevant complications. As a result, the risk of severe lung failure is reduced (33). Conversely, other researchers have reported an incremental risk of COVID-19 due to the suppression of the immune system due to HIV-1 infection (13). The use of ARVs during the COVID-19 pandemic is essential for maintaining health in HIV-1-infected individuals, particularly in older patients. Some studies have shown that older HIV-infected individuals (over 50-years-old) without ART are approximately 10 times more likely to develop SARS-CoV-2 than young HIV-infected patients who continue ART. According to other studies, the use of ART reduces morbidity and mortality of HIV-1-infected individuals with tuberculosis (34, 35).
The interim guidance for COVID-19 and patients with HIV infection indicates that elderly HIV-infected individuals are at the greatest risk of COVID-19 (36). Thus, the on-time availability of ARVs for HIV-infected individuals is critical during the COVID-19 pandemic (37). No COVID-19 was reported in a study on 199 HIV-infected patients using ritonavir/lopinavir or integrase inhibitors. However, 8/947 patients who used NRTIs and NNRTIs were infected with SARS-CoV-2 (11). In another study, HIV-infected individuals receiving TDF/FTC had a lower risk of developing COVID-19 than those receiving different ART (38). In another study, ART activity for HIV infection, including lopinavir/ritonavir, was effective against SARS or MERS (9). Remdesivir has been shown to be effective, too (39). Some studies have shown that HIV-1-infected individuals treated with ARVs, including tenofovir (TDF) or protease inhibitors, are less likely to become infected with SARS-CoV-2, with less severe COVID-19 (38). However, more studies are necessary to further elaborate on this condition. No significant association was observed in this research between HIV-1-infected individuals with or without COVID-19 and taking ARVs. In the present study, HIV-infected individuals admitted to the hospitals had the HIV viral load rate ranging from 0 to 11136 IU/mL and the CD4 count rate ranging from 65 to 1481 cells/µL. No differences were observed in the COVID-19 infection rate among individuals with or without HIV-1 infection. Global studies have shown that comorbidities and age may affect the COVID-19 severity and are not related to HIV infection (40, 41).
In northern Italy, HIV-infected individuals (3.4%) were infected with SARS-CoV-2 (41). In Spain, HIV-infected individuals (0.92%) were infected with SARS-CoV-2 (32). Another study in New York City reported 88 HIV-infected individuals with COVID-19 hospitalized. Additionally, a history of comorbidities and smoking was more prevalent in HIV-positive individuals than in HIV-negative patients (24). In Wuhan, the COVID-19 prevalence was reported among HIV-infected individuals (0.58%) (37). In Madrid, the COVID-19 prevalence was reported among HIV-infected individuals (1.8%) (12). Similarly, the COVID-19 prevalence among HIV-infected patients (0.6%) was reported in Wuhan, China (11). In Spain, out of 77,590 HIV-1-infected individuals receiving ARVs, 236 were infected with SARS-CoV-2. Among 236 patients diagnosed with COVID-19, hospitalization, ICU admission, and mortality were reported in 151 (64%), 15 (6%), and 20 (8%) patients, respectively (38). In China, a positive real-time PCR test was diagnosed for SARS-CoV-2 (62%) (42). The current study findings differ from those of some published studies. In other words, the COVID-19 prevalence was lower in this study than in some studies (42). However, the COVID-19 prevalence was higher in this study than in other studies (12, 32, 37, 41).
In Iran, out of 12,870 individuals, 2,968 hospitalized COVID-19 cases have been diagnosed. Also, 239 deaths have been reported. Moreover, the COVID-19 diagnosis rate was 66% in males. However, the current study findings do not support the previous research performed in Iran (43). In Iran, out of 161 suspected individuals of SARS-CoV-2 in the age range of 50 - 59 years, 102 showed positive real-time PCR test results; among them, a mortality rate of 15.6% was reported, accounting for 16 patients. Furthermore, two patients showed positive real-time PCR test results out of 13 suspected individuals with SARS-CoV-2 in the age range of 0 - 9 years. Moreover, no mortality was observed in these children (44). In Iran, out of 909 participants, 328 (36.08%) were diagnosed with COVID-19 (45). However, the COVID-19 prevalence in this study was lower than the results reported in some studies conducted in Iran (45). This study indicated the COVID-19 infection rate in HIV-1-infected individuals (n = 12, 7.7%), unlike previous studies performed among the Iranian population (43, 45). In the present study, the COVID-19 diagnosis rate was higher in females (n = 8, 17.4%) than males (n = 4, 3.7%). The current study findings are consistent with a previous study conducted in Iran, showing that the COVID-19 prevalence was higher in women than in men. The incidence of COVID-19 in females and males was reported to be 185 (20.35%) and 143 (15.73%), respectively (45). Out of the nine hospitalized children in Iran, three positive real-time PCR tests for SARS-CoV-2 were reported (46).
The clinical and demographic characteristics of HIV-1-infected individuals with a positive SARS-CoV-2 real-time PCR result in this study differed from those reported in another study conducted in Iran; patients showed fewer symptoms such as fever and dry cough in this study than in another study (47). Moreover, there was no association between the COVID-19 diagnosis and CD4 cell counts in HIV-1-infected individuals in this study. According to our findings, HIV-infected individuals are likely to be at a similar risk for SARS-CoV-2 in clinical manifestations as others in the community. Also, ARVs do not appear to be effective against COVID-19. In this study, HIV-infected patients approximately had a well immunological condition; thus, an incremental risk of COVID-19 was not associated with a low CD4 + count. The present study showed that the risk of developing COVID-19 in HIV-infected individuals was similar to the general population.
In this evaluation, volunteers had various types of blood groups. Out of 12 (7.7%) positive real-time PCR results for SARS-CoV-2, four, three, three, and two HIV-1-infected individuals were identified with O+ blood, A+ blood, B+ blood, and AB+ blood, respectively. Out of the 10 (6.5%) positive results for anti-COVID Ab (IgG) against SARS-CoV-2, the numbers of HIV-1 infected individuals with O+ blood, O- blood, and A+ blood were six, two, and two, respectively. There was no association between the COVID-19 diagnosis and different blood types in HIV-1-infected individuals in this research. However, some studies have shown that people with O blood are less susceptible to COVID-19 (48, 49).
5.1. Conclusions
The present study's primary purpose was to investigate the COVID-19 prevalence among HIV-1-infected patients, focusing on laboratory and epidemiological characteristics of COVID-19 in the Iranian population. However, during the COVID-19 pandemic, the screening and identification of HIV-1-infected individuals were limited. The access to the number of recent HIV-1-infected individuals was affected by COVID-19 limitations. Despite these limitations, this study elaborated on the characteristics of HIV-1-infected individuals with COVID-19 in the Iranian population. Only 12 (7.7%) HIV-1-infected patients were positive for the SARS-CoV-2 real-time PCR test. In this study, females (n = 8, 17.4%) had a higher COVID-19 infection rate than males (n = 4, 3.7%). Nevertheless, males (n = 8, 7.3%) had higher anti-COVID-19 Ab (IgG) than females (n = 2, 4.3%) in total cases (n = 10, 6.5%). During the COVID-19 outbreak, more studies are needed to examine HIV-1-infected individuals' health conditions worldwide.