1. Background
Helicobacter pylori is the most common causative agent for gastric infections, especially in the developing world. Helicobacter pylori was discovered in 1982 in the human stomach (1). Despite colonization for years, this microaerophilic Gram-negative rod runs an asymptomatic course in most patients. Helicobacter pylori transmits gastric infection by fecal-oral, oral-oral, zoonotic, water, and gastric-oral pathways (2). Environmental factors linked with its colonization in the stomach may include but are not limited to obesity, alcohol intake, suboptimal living conditions, nutritional deficiencies, and smoking (2). Surface epithelial degeneration, along with neutrophil and monocyte infiltration, leads to pan-gastritis, multifocal atrophy, and intestinal metaplasia, thus predisposing an individual to future peptic ulceration (3).
Sleeve gastrectomy (SG) is typically employed for weight loss by permanently removing about 15 - 20% portion of a large-sized stomach by surgery, leading to reduced food intake (4). Nowadays, it is considered a rapid weight loss method and is opted by choice by both genders (5). Recent literature reports that the outcome of sleeve gastrectomy patients is affected by the presence or absence of H. pylori (6). However, it may not be a potential prognostic determinant in certain groups of patients. It is also suggested that H. pylori eradication should be given before sleeve gastrectomy, even in patients with mild clinical symptoms (1). However, this practice is clinically debatable in our country. In addition, correlating patients' clinical condition with the tests having high sensitivity and specificity for detecting H. pylori leads to an additional financial burden on the patients, hence seldom ordering. Thus, this study was designed to detect H. pylori in sleeve gastrectomy specimens by utilizing different laboratory methods and correlating the burden of the morbidity with the surgical outcome in these patients.
2. Objectives
This study was designed to detect the frequency of H. pylori by different techniques in sleeve gastrectomy specimens of obese patients with minimal or no symptoms suggestive of gastritis.
3. Methods
3.1. Patients and Sampling
This longitudinal study was carried out at the Farooq Hospital Westwood Lahore, Pakistan, and the Department of Morbid Anatomy and Histopathology and Resource Lab at the University of Health Sciences, Lahore, Pakistan, from February 2021 to September 2021. Sample size (n) of 80 was calculated by the following formula keeping the confidence level (1 - α/2) equal to 95% and the margin of error (d) equal to 10% and assuming 71% prevalence (P) of H. pylori in sleeve gastrectomy (7). A total of 80 cases who underwent sleeve gastrectomy were recruited under strict inclusion criteria.
Inclusion criteria were patients of adult age group (> 18 years) from both genders and body mass index (BMI) ≥ 30 kg/m2 as per the World Health Organization (WHO) definition (8). Patients having associated chronic comorbid conditions like diabetes, hypertension, cardiovascular diseases, previous gastric surgery, and second bariatric surgery were excluded. Specimens with inadequate tissue for histological reporting were also excluded. The relevant clinical history and preoperative complete blood counts, blood HbA1C, sugar levels, renal and liver function tests, and coagulation profile were analyzed for the recruitment of cases after obtaining written informed consent from the patients.
3.2. Rapid Urease Test
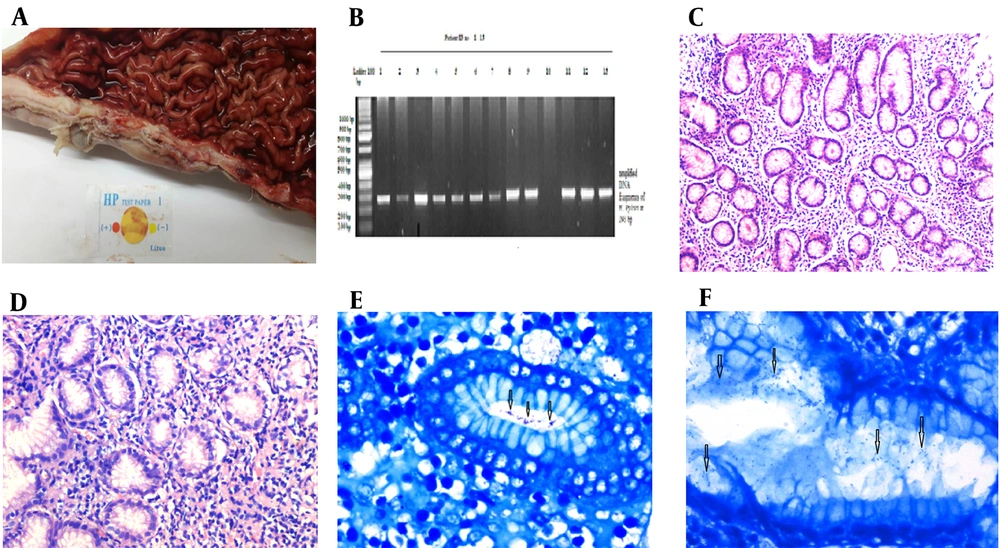
Rapid urease test (RUT) was performed at the bedside on sleeve gastrectomy biopsies. For the RUT (test card method, Lituo Biotechnology, China), cherry color was taken as positive for H. pylori, whereas yellow color was taken as negative (Figure 1A). Each biopsy specimen was portioned equally for histopathological and molecular analysis. After the orientation of specimens, fixation in 10% neutral formaldehyde was done for histological analysis. Approximately 4 - 5 µm sections were cut perpendicular to the surface, and H & E and modified Giemsa stains were used to detect H. pylori.
The organism was detected under the microscope by the presence of curved, rod-shaped cells within the gastric pits and the mucosal folds. At least two histopathologists independently analyzed the biopsies microscopically regarding the grading of chronic gastritis according to the updated Sydney system. Biopsies were observed for H. pylori density, neutrophil infiltration, degree of chronic inflammation, glandular hyperplasia, glandular atrophy, intestinal metaplasia, dysplasia, surface erosions, lymphoid aggregates, and follicles (9-11). Dixon visual analog scale was used to grade variables as mild, moderate, and severe (9, 10). Helicobacter pylori colonization was subjectively scaled as none, mild (few microorganisms), moderate (microorganism distributed at multiple foci), and severe (complete or near-complete surface layering) (9-11). Inter-rater reliability was calculated by Cohen's Kappa coefficient (K) following Fleiss's method (12).
A, Photograph showing the gross appearance of sleeve gastrectomy specimen and rapid urease test; B, Amplified fragments of Helicobacter pylori DNA by PCR in sleeve gastrectomy specimen at 293 bp and ladder 100 bp; C, Photomicrograph showing mild chronic gastritis in sleeve gastrectomy specimen (200X, H & E stain); D, Photomicrograph showing moderate-severe chronic gastritis in sleeve gastrectomy specimen (200X, H & E stain); E, Photomicrograph showing mild colonization of H. pylori in sleeve gastrectomy specimen (400X, Modified Giemsa stain); F, Photomicrograph showing moderate colonization of H. pylori in sleeve gastrectomy specimen (400X, Modified Giemsa stain).
3.3. Molecular Detection
For molecular analysis, the other half of the biopsy specimen was frozen at -80°C until the extraction of deoxyribonucleic acid (DNA) for H. pylori was carried out with the help of a DNAsy kit (Qiagen, Cat. No. 56404) following the manufacturer's recommended procedure. Polymerase chain reaction (PCR) was carried out in 16 µL reaction mixtures comprising 2 µL of genomic DNA, 10 µL of PCR master mix, 0.5 µL of forward primer working solution, 0.5 µL of reverse primer working solution, and 3 µL of nuclease-free water. The specific primer for H. pylori species-specific antigen (SSA) gene were as follows: sense, "TGGCGTGTCTATTGACAGCGAGC" and antisense, "CCTGCTGGGCATACTTCACCATG" (13-15).
The quality and quantity of extracted DNA were determined by a NanoDrop spectrophotometer (Thermo Scientific NanoDrop 2000). The PCRs were run in a thermocycler with an automated iQ5 (Bio-Rad, USA). In the PCR, initial denaturation was carried out for three minutes at 95°C and 35 cycles of denaturation at the same temperature for one minute. The annealing was carried out at 68°C and 72°C for one minute. The seven-minute extension step for the final and complete extension of the PCR was carried out. After completing every PCR for H. pylori, the product was run on agarose gel electrophoresis to observe the amplification of H. pylori. The gel was examined using Gel Doc XR+ system (Bio-Rad, USA), and the picture was taken and saved for analysis. The amplified DNA of H. pylori was observed at 293 bp, compared to the 100 bp ladder (Figure 1B). Also, H. pylori strain G27 was taken as a positive control, whereas nuclease-free water was run as a negative control for H. pylori with each batch of PCR (16). The patients were discharged on day 3 and were followed up at an interval of one month until six months. Each patient with positive H. pylori result was prescribed 40 mg OD of proton pump inhibitor therapy for two months.
3.4. Statistical Analysis
The data were analyzed using statistical package for the social sciences (SPSS) version 22.0. Mean and standard deviation (SD) were generated for continuous clinical, demographic, and anthropometric variables. Categorical variables were represented as frequencies and percentages. Continuous were expressed as mean ± SD. Histological grading of chronic gastritis, H. pylori colonization, and H. pylori positivity were expressed as frequency and percentage. A comparison between symptomatic and asymptomatic patients was made using a student t-test for continuous variables. The Pearson chi-square test was applied to determine the significant differences in the proportion of chronic gastritis grading and H. pylori colonization between symptomatic and asymptomatic patients. The Pearson chi-square test or Fisher's exact test was applied to find the relationship between gastritis grades, H. pylori colonization, and BMI. Bonferroni post hoc test was used to compare the pre-and post-surgical mean BMI in both groups. All analyses were two-tailed, and a P-value ≤ 0.05 was considered significant. Using OpenEpi software, the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of rapid urease test and modified Giemsa stain were calculated compared to PCR as the gold standard.
4. Results
A total of 80 subjects undergoing sleeve gastrectomy were recruited. There were 48 (60%) females and 32 (40%) males with a gender ratio (F: M) of 1.5: 1. The mean age of the study population was 39.0 ± 10.05 years. The mean age of male subjects was 39.0 ± 10.04 years, while that of female subjects was 39.0 ± 10.06 years. The mean BMI of the enrolled subjects was 39.9 ± 2.8 kg/m2 (range: 35.1 - 44.7 kg/m2) (Table 1). The mean BMI of asymptomatic subjects was 40.6 ± 2.4 kg/m2, while that of symptomatic subjects was 36.3 ± 1.2 kg/m2. Gross examination of sleeve gastrectomy was carried out by measuring length and diameter. The mean length was 22.0 ± 6.7 cm, and the diameter was 6.3 ± 2.4 cm. Histopathological examination revealed that most specimens had submucosal lymphocytic and plasmacytic infiltrates. Active inflammation was observed in 10.4% (n = 7) of asymptomatic and 69.2% (n = 9) of symptomatic cases, which indicated the presence of neutrophils in the mucosal lining (Table 2). Surface erosion was observed in 9% (n = 6) of asymptomatic and 76.9% (n = 10) of symptomatic cases. Intestinal metaplasia and dysplastic changes were not observed in any of the biopsies.
| Variables | Asymptomatic; n = 67 (83.7%) | Symptomatic; n = 13 (16.3 %) | P-Value |
|---|---|---|---|
| Age (y) | Gender = 0.902, age = 0.847 | ||
| Female | 39.0 ± 10.06 | ||
| Male | 39.0 ± 10.04 | ||
| BMI (kg/m2) | 40.6 ± 2.4 | 36.3 ± 1.2 | < 0.001 |
| Chronic Gastritis | 0.003 | ||
| Total | 38 (56.7 ) | 13 (100 ) | |
| Mild | 31 (81.6 ) | 8 (61.5 ) | |
| Moderate to severe | 7 (18.4 ) | 5 (38.5) | |
| Activity | 7 (10.4 ) | 9 (69.2 ) | |
| Intestinal metaplasia | None | None | |
| Helicobacter pylori | |||
| RUT | 18 (47.3 ) | 12 (92 ) | < 0.001 |
| Modified Giemsa | 18 (47.3 ) | 12 (92 ) | < 0.001 |
| PCR | 23 (60.5 ) | 13 (100 ) | < 0.001 |
| H. pylori Colonization | |||
| None | 20 (52.6 ) | None | |
| Mild | 11 (61 ) | 7 (53.7 ) | < 0.001 |
| Moderate to severe | 7 (39 ) | 5 (38.5 ) |
a Kappa coefficient result = 0.75 (Kappa analysis of blinded colonization by a pathologist).
b Values are expressed as No. (%) or mean ± SD.
| Chronic Gastritis | Mild | Moderate-Severe | Total |
|---|---|---|---|
| Asymptomatic | |||
| Histologically confirmed | 31 (81.6) | 07 (18.4) | 38 (56.7) |
| Activity | 03 (4.4) | 04 (6.0) | 07 (10.4) |
| Helicobacter pylori positive | 19 (50.0) | 04 (10.5) | 23 (60.5) a |
| Symptomatic | |||
| Histologically confirmed | 08 (61.5) | 05 (38.5) | 13 (16.3) |
| Activity | 06 (46.2) | 03 (23.0) | 09 (69.2) |
| H. pylori positive | 08 (61.5) | 05 (38.5) | 13 (16.3) b |
a Out of 23 positive Helicobacter pylori in asymptomatic subjects, 18 were positive on RUT and modified Giemsa staining, while additional five cases were positive on PCR.
b Out of 13 positive H. pylori cases, 12 were positive on RUT and modified Giemsa staining, while an additional one case was positive on PCR.
Most of the patients (n = 67, 83.7%) were clinically asymptomatic, while 10% (n = 8) had mild and 6.3% (n = 5) had moderate to severe gastritis symptoms. Rapid urease test was positive in 92% (n = 12) of symptomatic and 47.3% (n = 18) of asymptomatic cases. Of the asymptomatic patients, 56.7% (n = 38) biopsies showed chronic gastritis. A total of 81.6% (n = 31) of these patients had mild chronic gastritis, while 18.4% (n = 7) had moderate chronic gastritis. Modified Giemsa staining revealed positive H. pylori in 47.3% (n = 18) of biopsies. The H. pylori colonization was mild in 11 (61%) and moderate in seven (39%) biopsies. None of the biopsies showed severe colonization according to Fleiss's criteria (Table 1) (12). The PCR analysis of all tissue specimens revealed the amplification of H. pylori DNA in an additional 13.2% (n = 5) of biopsies that were negative on conventional methods. Hence, 23 (60.5%) asymptomatic cases had positive H. pylori on combined histological and molecular analyses (Table 2).
A total of 13 (16.3%) patients were clinically symptomatic. Among them, mild gastritis (Figure 1C) was seen in the biopsies of 61.5% (n = 8) of cases, while moderate to severe gastritis was seen in 38.5% (n = 5) of patients (Figure 1D). The rapid urease test (Figure 1A) was positive in 92.3% (n = 12) of symptomatic cases. Modified Giemsa staining revealed positive H. pylori in these 12 (92.3%) cases. The H. pylori colonization was mild in seven (53.8%) (Figure 1E) and moderate in five (38.5%) biopsies (Figure 1F), while none showed severe colonization according to Fleiss's criteria (Table 1) (12). The PCR analysis of all tissue specimens revealed the amplification of H. pylori DNA in an additional case that was negative on conventional methods. Hence, all symptomatic cases had positive H. pylori on combined histological and molecular analyses (Table 2).
The Pearson Chi-square test or Fisher's exact test was applied to observe the relationship between the grades of gastritis, the intensity of H. pylori colonization, and BMI. The mean BMI was significantly higher (P = 0.001) in mild chronic gastritis patients than in those with moderate-severe gastritis. Also, H. pylori positivity was observed in 75% of cases with moderate-severe chronic gastritis and 69% with mild chronic gastritis (P = 0.701), which may depict that H. pylori positivity increases with the increasing grades of chronic gastritis. Moreover, H. pylori colonization was found in all biopsies (from both symptomatic and asymptomatic cases) that showed moderate-severe grading of chronic gastritis, whereas 46.6% of biopsies with mild chronic gastritis showed positive colonization (P < 0.001).
Patients were followed up for six months, and no adverse post-surgical event occurred in any enrolled cases. After six months, a serum sample of H. pylori-positive patients was taken, and H. pylori positivity was tested with a rapid antibody-based (IgM, IgG) immune-chromatographic test (one step H. pylori Device, Acu-Check - Lot # HP1911036). The test results of all previously positive patients were negative. Bonferroni post hoc testing revealed that the mean BMI six months after surgery was significantly reduced to 22.1 ± 1.6 kg/m2 in symptomatic patients and 26.7 ± 2.2 kg/m2 in asymptomatic patients (P = 0.021). The diagnostic accuracy of the rapid urease test and modified Giemsa stain showed equal sensitivity of 83.3% and specificity of 100% (Table 3).
| Sr No. | Stain | Gold Standard Technique | Sensitivity = (A/A + C) × 100 (%) | Specificity = (D/B + D) × 100 (%) | Positive Predictive Value = (A/A + B) × 100 (%) | Negative Predictive Value = (D/D + C) × 100 (%) | Diagnostic Accuracy = (A + D/A + D + C) × 100 (%) |
|---|---|---|---|---|---|---|---|
| 1 | RUT | PCR | 83.3 | 100 | 100 | 71.4 | 88.2 |
| 2 | Modified Giemsa | PCR | 83.3 | 100 | 100 | 71.4 | 88.2 |
5. Discussion
Limitations of culture-based studies have inspired pathologists worldwide to explore highly sensitive nucleic acid-based assays for microbial detection in this modern era. Polymerase chain reaction (organism-based) has proven the most promising molecular modality in terms of rapidity, overall cost-effectiveness, and accuracy in diagnosing H. pylori, even in resource-constrained settings. This study also depicts a high diagnostic accuracy of PCR-based H. pylori detection compared with conventional methods in biopsies of subjects undergoing sleeve gastrectomy for weight loss. In resource-constrained countries like Pakistan, where molecular analysis is not offered even to afford patients with gastritis because of high cost, the clinical diagnosis mainly relies on rapid urease or urea breath tests in clinical set-ups, while histology remains the ultimate diagnostic modality in such cases.
The mean age of the study subjects in the present study was 39.0 ± 10.05 years which is comparable to Serin et al. (1), who reported the mean age of 37.53 ± 9.95 years, thus depicting a younger age population having obesity-related issues and opting for surgical solutions. The present study reported more females affected than males (F: M) 1.5: 1, concordant with other studies reporting higher female prevalence (10, 17). The mean BMI of subjects in the present study was 39.9 ± 2.8 kg/m2, whereas higher mean BMIs of 42.69 ± 7.7 kg/m2 and 45.5 ± 8.5 kg/m2 have been reported in other studies (1, 18). Some researchers reported that adequate clinical data and preoperative endoscopic findings help determine outcomes in sleeve gastrectomy patients. Helicobacter pylori may predict worse outcomes in sleeve gastrectomy patients (7, 10). On the other hand, it is reported that eradication therapy for H. pylori is not essential for asymptomatic patients (10). According to the World Health Organization, more than 50% of the human population is infected with H. pylori (19), whereas 80% remain asymptomatic (7). A high number of patients undergoing sleeve gastrectomy remain asymptomatic (7). Therefore, current European guidelines recommend gastric endoscopy prior to bariatric surgery in both symptomatic and asymptomatic patients for better patient outcomes (20).
In the present study, microscopic evidence of H. pylori colonization with chronic gastritis was observed in more than 60% of asymptomatic subjects. A much lower frequency (38.8%) was reported in another study in which Warthin-Starry stain was used in sleeve gastrectomy biopsies of asymptomatic cases (10). Keren et al. (21) also reported only (21%) of asymptomatic cases that turned out positive on gastric biopsies using Giemsa stain. This may be attributed to both host and microbial variations across different geographic settings that determine immunologic response to colonization. Regarding the sensitivity of the rapid urease test, there exist conflicting reports. However, it is still termed the most sensitive and reliable method for H. pylori detection in resource-limited economies (7).
Rapid urease test yielded 47.3% positive H. pylori from asymptomatic cases, whereas modified Giemsa stain reported similar results of positive rods in biopsies. These results are comparable with Pintar et al. (7), who reported 63% results for RUT and 76% for Giemsa stain. Regarding the diagnostic sensitivity, specificity, positive predictive value, and negative predictive value of modified Giemsa stain, the results of the present study (Table 3) are comparable with other studies (22). The sensitivity and specificity of rapid urease are also comparable with Aziz et al. (11), whereas Akanda and Rahman (23) reported higher values (96.6%) than the current study. The association between grades of chronic gastritis, H. pylori colonization, and diagnostic accuracy of rapid urease test and modified Giemsa stain is comparable with Aziz et al. (11) and Akanda and Rahman (23). Modified Giemsa stain is considered a simple, readily available, and sensitive test with good diagnostic yield (24), but when compared with molecular analysis, it shows lower sensitivity (42.3 %), keeping PCR as the gold standard (25).
Molecular detection methods such as PCR are considered sensitive and gold standard compared to histology by different researchers (26). According to Patel et al. (26), some false positive and negative results could arise by PCR methodology. The PCR methods may be less sensitive because of sample contamination by PCR products, inadequate disinfection of endoscopy, low number of bacteria, or detection of non-pylori Helicobacter due to genetic sharing and presence of PCR inhibitors (26). According to de Martel et al. (27), histology is more accurate than the PCR for H. pylori detection. Multiple genes are used for the detection of H. pylori, such as SSA, urease A, glmM, CagA, and Vac A. Literature reveals that the SSA gene shows promising results in detecting H. pylori in gastric biopsies (28, 29) with high sensitivity and specificity compared to the other standard genes tested simultaneously (15). Smith et al. (15) reported 44% SSA gene positivity with a 100% positive predictive value.
Ribeiro et al. (28) performed real-time PCR on 81 cases that were negative on RUT, histopathology, and culture and reported 19.8% (16/81) positivity after RT-PCR assay with the SSA gene. Helicobacter pylori was positive on PCR in 60.5% of biopsies in the present research, which is quite comparable to the findings of Šebunova et al. (30), who reported 64.7% positivity by PCR analysis. However, Shetty et al. (31) reported microscopy with Giemsa stain as a more reliable test (54.7%) compared to the sensitivity of PCR (54.5%) and rapid urease test (4.9%). Therefore, test results may vary due to different techniques, troubleshooting methods, and detection kits used in various clinical laboratories.
In the present study, molecular detection showed promising results as the most sensitive method for detecting H. pylori in negative samples, enabling better clinical management. However, diagnosis based on PCR can be considered a gold standard by designing H. pylori-specific primers with more than one conserved gene targeted. The clinical outcome in all patients undergoing sleeve gastrectomy was uneventful, which may be attributed to incorporating H. pylori testing methods in surgically removed gastric tissues and prescribing appropriate post-surgical H. pylori treatment regimens to patients with positive results.
5.1. Conclusions
The experimental results of the present study indicated that persistent obesity might lead to the colonization of H. pylori which remains primarily asymptomatic. More sensitive techniques for detecting H. pylori may be employed in resource-constrained settings for better patient outcomes and to minimize complications after sleeve gastrectomy. Larger prospective studies may be carried out to explore potential pathogenic links between obesity-related chronic gastritis and the presence of H. pylori.