1. Background
Urinary tract infection (UTI), which involves many organs of the body, is one of the most common diseases in hospitals and the community (1). They can affect adults, youth, and children (2, 3). Urinary tract infections affect women much more frequently than men because of the anatomical configuration of the female genital tract, particularly the proximity of the urinary meatus to the anus (4). Urinary tract infections represent a major expensive (5) and common public health problem worldwide due to their high prevalence, difficulties associated with their management, and their complications (6). This concerns all outpatients and inpatients (7). Every year, above 200 million people are globally diagnosed with UTI, accounting for the global economy above $6 billion (6, 7).
The most isolated specie is Gram-negative (enterobacteria) (3), such as Escherichia coli, Enterobacter spp., Klebsiella spp. (8), Pseudomonas aeruginosa, and Serratia marcescens with high resistance rates (9). Among the strains, E. coli, Klebsiella pneumoniae, and Enterobacter cloacae are three significant pathogens involved in UTIs with important resistance phenotypes (10). These bacterial species have identified the most developed resistance mechanisms in enterobacteria. The World Health Organization (WHO) continues to raise the alarm about the severe handicap of antimicrobial resistance in managing infections such as UTIs. Some evidence shows an increasing resistance to conventional drugs among urinary tract pathogens (11, 12). The high rates of resistance to antimicrobials often used to treat common infections such as UTIs indicate the limited number of effective ways to control these infections. The situation is more alarming because infections induced by resistant bacteria often lead to a prolonged disease state and an increased mortality rate (9).
According to recent statistics reported by the WHO, the rate of resistance to ciprofloxacin used to treat UTIs ranged from 8.4% to 92.9% in 33 countries (13). Enterobacter cloacae isolates involved in UTIs have been characterized as multidrug-resistant for many years (14). Due to various intrinsic and acquired resistance mechanisms, it possesses; E. cloacae presents a real therapeutic challenge (7). Many studies have indicated antimicrobial resistance in E. cloacae strains isolated from UTIs (7, 15). The antimicrobial resistance of bacteria is one of the major problems in the world (3). However, few studies have focused on detecting the genetic elements of resistance in this bacterium (16). The present study was to fill this gap and provide usable scientific data on the resistance level of E. cloacae isolates in Shahrekord, Iran. This study would improve the treatment and management of UTIs because of using the multidrug-resistant strains of E. cloacae.
2. Objectives
This study aimed to provide information about the molecular profile of E. cloacae strains isolated from UTIs.
3. Methods
3.1. Study Setting
In this cross-sectional study, about 786 urine samples from male and female patients of different age ranges and history of UTI, who referred to the medical diagnostic laboratories in Shahrekord were collected from June 2019 to February 2020. Informed consent was obtained from the patients before collecting the samples. Moreover, ethical approval was obtained from the ethics committee. Bacterial strains were identified based on their culture and biochemical characteristics (6).
3.2. Antibiotic Susceptibility Study
The antibiotic sensitivity of the isolated strains was determined using the Kirby Bauer disk diffusion method. The antibiotic disc (Padtanteb, Iran) containing the following antibiotics was used: Amikacin (30 μg/AN30), kanamycin (K/30 μg), erythromycin (E15/15 μg), tetracycline (TE30/30 µg), gentamicin (GM10/10 μg), chloramphenicol (C) ampicillin (AM10/10 µg), ceftriaxone (CRO30/30 μg), ciprofloxacin (CP5/30 μg), cephalothin (CF30/30 μg), imipenem (IPM10/10 μg), nitrofurantoin (FM300/10 μg), nalidixic acid (NA30/30 μg), norfloxacin (NOR10/30 µg), and co-trimoxazole (SXT25/30 μg). The obtained inhibition zone diameters (IZDs) were recorded and interpreted according to the CLSI guidelines (6).
3.3. DNA Extraction and PCR Reactions
The DNA extraction kit (CinnaGen, Iran) was used according to the manufacturer’s instructions to obtain Genomic DNA from bacterial colonies. The selected isolates were subjected to multiplex PCR using previously described Enterobacter primers (CinnaGen, Iran) and conditions set by Tajbakhsh et al. (17) (Table 1).
| Primer Sequences (5′-3′) |
|---|
| F: ATGTCTGGGAAACTGCCTGATG |
| R: CGGGTAACGTCAATAGACAAGG |
3.4. Detection of Antibiotic Resistance Genes
PCR was performed for antibiotic resistance genes using the primers (CinnaGen, Iran) described in Table 2 (18-22). Hybridization temperatures and PCR conditions vary from gene to gene. The PCR conditions for each gene are presented in Table 3. DNA sequencing was performed with the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST) to confirm resistance gene variants.
| Gene and Primer Sequence (5’-3’) | Size of Product (bp) |
|---|---|
| qnr A | 360 |
| F: CAGCAAGAGGATTTCTCACG | |
| R: AATCCGGCAGCACTATTACTC | |
| qnr B | 488 |
| F: GGCTGTCAGTTCTATGATCG | |
| R: GAGCAACGATGCCTGGTAG | |
| qnr S | 428 |
| F: GCAAGTTCATTGAACAGGGT | |
| R: TCTAAACCGTCGAGTTCGGCG | |
| ant (2)Ia | 572 |
| F: CGCCGTGGGTCGATGTTTGATG | |
| R: TTTTCCGCCCCGAGTGAGGTG | |
| ant (3)Ia | 245 |
| F: TCGACTCAACTATCAGAGG | |
| R ACAATCGTGACTTCTACAGCG | |
| aac(3)IIa | 563 |
| F: GCAATAACGGAGGCGCTTCAAAA | |
| R: TTCCAGGCATCGGCATCTCATACG | |
| Sul 1 | 433 |
| F: CGGCGTGGGCTACCTGAACG | |
| R: GCCGATCGCGTGAAGTTCCG | |
| Bla SHV | 231 |
| F: AAG ATC CAC TAT CGC CAG CAG | |
| R: ATT CAG TTC CGT TTC CCA GCG G | |
| Bla CITM | 850 |
| F: GGT TAA AAA ATC ACT GCG TC | |
| R: TTG GTG ACG ATT TTA GCC GC | |
| tet A | 927 |
| F: GTAATTCTGAGCACTGTCGC | |
| R: CTGCCTGGACAACATTGCTT | |
| tet B | 659 |
| F: TTGGTTAGGGGCAAGTTTTG | |
| R: GTAATGGGCCAATAACACCG |
| Gene | PCR Program | PCR Condition |
|---|---|---|
| qnr A | 1 cycle: 94°C---6 min; 33 cycles: 95°C---70 s, 55°C---65s, 72°C---90 s, 1 cycle: 72°C---8 min. | 5 μL PCR buffer 10X, 2.5 mm MgCl2, 200 μM dNTP (Fermentas), 0.5 μm of each primers F & R. |
| qnr B | ||
| qnr S | 1 cycle: 95°C---5 min; 31 cycles: 95°C---45s, 59°C---60s, 72°C---60s, 1 cycle: 72°C---5 min. | |
| ant (2)Ia | 1 cycle: 95°C---4 min; 30 cycles: 95°C---45s, 58°C---60s, 72°C---40s, 1 cycle: 72°C---5min. | |
| ant (3)Ia | ||
| aac(3)IIa | 1 cycle: 94°C---5 min; 32 cycles: 94°C---60 s, 55°C---60 s, 72°C---2 min, 1 cycle: 72°C---10 min. | |
| Sul 1 | 1 cycle: 94°C---5 min; 32 cycles: 94°C---60s, 60°C---60s, 72°C---2 min, 1 cycle: 72°C---5min. | |
| Bla SHV | 1 cycle: 95°C---4min; 30 cycles: 94°C---45s, 59°C---60s, 72°C---40s, 1 cycle: 72°C---5min. | |
| Bla CTX-M | ||
| tet A | 1 cycle: 95°C---5min; 32 cycles: 94°C---60s, 59°C---60s, 72°C---2min, 1 cycle: 72°C---10min. | |
| tet B |
3.5. Biofilm Formation Assay
The ability of the isolates to form biofilms was investigated by a method previously described by Naves et al. (23). It is based on the assay in minimum and enriched media of E. cloacae strains and the mathematical determination of the patterns and quantity of biofilm formed.
3.6. Statistical Analysis
Descriptive analyses, including the mean value and variability (standard deviation) and descriptive tables, were used. Chi-squared and Fisher’s exact tests were used to evaluate the significance of differences between the different parameters evaluated with EpiData software (P < 0.05).
4. Results
This study aimed to characterize the molecular level of E. cloacae strains involved in the urinary tract of infected patients. To this end, 65 E. cloacae were isolated from 786 urine samples obtained from medical diagnostic laboratories, Shahrekord, Iran. Of the 65 E. cloacae isolates, 55 isolates were female, and 10 isolates were male. For each isolate, characteristic tests were performed.
4.1. Antibiotic Susceptibility Study
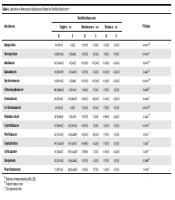
The results of the sensitivity test of the strains to different antibiotics are presented in Table 4. This table shows that half of the E. cloacae isolates are multidrug-resistant to ampicillin, tetracycline, amikacin, kanamycin, erythromycin, chloramphenicol, gentamicin, co-trimoxazole, and nalidixic acid, which are antibiotics from various classes. Resistance to imipenem, ceftriaxone, ciprofloxacin, cephalothin, and norfloxacin was also observed for above 30% of the strains. Only 20% of the strains were sensitive to imipenem. The highest resistance percentage was observed for co-trimoxazole (84.62%), ampicillin (76.93%), and tetracycline (73.85%), respectively. On the other hand, the resistance percentages were only 23.08% for Nitrofurantoin and 30.77% for imipenem and cephalothin.
| Antibiotic | R | I | S |
|---|---|---|---|
| Ampicillin | 50 (76.93) | 10 (15.38) | 5 (7.69) |
| Tetracycline | 48 (73.85) | 11 (16.92) | 6 (9.23) |
| Amikacin | 45 (69.23) | 15 (23.08) | 5 (7.69) |
| Kanamycin | 40 (61.54) | 15 (23.08) | 10 (15.38) |
| Erythromycin | 43 (66.16) | 12 (18.46) | 10 (15.38) |
| Chloramphenicol | 40 (61.54) | 10 (15.38) | 15 (23.08) |
| Gentamicin | 35 (53.84) | 15 (23.08) | 15 (23.08) |
| Co-Trimoxazole | 55 (84.62) | 5 (7.69) | 5 (7.69) |
| Nalidixic Acid | 45 (69.23) | 15 (23.08) | 5 (7.69) |
| Ciprofloxacin | 30 (46.15) | 15 (23.08) | 20 (30.77) |
| Norfloxacin | 25 (38.46) | 20 (30.77) | 20 (30.77) |
| Cephalothin | 20 (30.77) | 15 (23.08) | 30 (46.15) |
| Ceftriaxone | 25 (38.46) | 20 (30.77) | 20 (30.77) |
| Imipenem | 20 (30.77) | 25 (38.46) | 20 (30.77) |
| Nitrofurantoin | 15 (23.08) | 17 (26.15) | 33 (50.77) |
a Values are expressed as No. (%).
4.2. Biofilm Production Study
Regarding the biofilm production by E. cloacae, above half of the E. cloacae strain isolates (53.85%) were strongly involved in biofilm production. Moreover, 53.85% had high biofilm production activity, 30.77% had moderate production, and 15.38% had low production.
4.3. Antibiotic Resistance Based on Biofilm Production
The relationship between biofilm production and the antibiotic resistance of E. cloacae strains is presented in Table 5. According to this table, the majority of strains with a high capacity for biofilm production were resistant to ampicillin (100%), amikacin (94.29%), tetracycline (97,14%), erythromycin (97.14%), co-trimoxazole (100%) (P < 0.05), and the half of these strains were resistant to most of the used antibiotic. Moreover, half of the strains with moderate biofilm production reaction showed resistance to several classes of antibiotics, including ampicillin (75%), tetracycline (55%), amikacin, erythromycin (50%), co-trimoxazole (55%), ciprofloxacin (70%), and ceftriaxone (85%). Antibiotic resistance was observed for strains with low biofilm production capacity, however, to a lesser extent.
| Antibiotic | Biofilm Reaction | P-Value | |||||
|---|---|---|---|---|---|---|---|
| High n = 35 | Moderate n = 20 | Weak n = 10 | |||||
| R | S | R | S | R | S | ||
| Ampicillin | 35 (100) | 0 (0) | 15 (75) | 5 (25) | 5 (50) | 5 (50) | 0.000 b |
| Tetracycline | 34 (97.14) | 1 (2.86) | 11 (55) | 9 (45) | 3 (30) | 7 (70) | 0.000 c |
| Amikacin | 33 (94.29) | 2 (5.71) | 10 (50) | 10 (50) | 6 (60) | 4 (40) | 0.001 b |
| Kanamycin | 30 (85.71) | 5 (14.29) | 15 (75) | 5 (25) | 6 (60) | 4 (40) | 0.243 b |
| Erythromycin | 34 (97.14) | 1 (2.86) | 10 (50) | 10 (50) | 6 (60) | 4 (40) | 0.000 b |
| Chloramphenicol | 29 (82.86) | 6 (17.14) | 13 (65) | 7 (35) | 7 (70) | 3 (30) | 0.298 b |
| Gentamicin | 25 (71.43) | 10 (28.57) | 12 (60) | 8 (40) | 6 (60) | 4 (40) | 0.624 c |
| Co-Trimoxazole | 35 (100) | 0 (0) | 11 (55) | 9 (45) | 7 (70) | 3 (30) | 0.000 b |
| Nalidixic Acid | 32 (91.83) | 3 (8.57) | 15 (75) | 5 (25) | 8 (80) | 2 (20) | 0.256 b |
| Ciprofloxacin | 10 (28.57) | 25 (71.43) | 14 (70) | 1 (30) | 5 (50) | 5 (50) | 0.000 c |
| Norfloxacin | 20 (57.14) | 15 (42.86) | 12 (60) | 8 (40) | 7 (70) | 3 (30) | 0.765 c |
| Cephalothin | 19 (54.29) | 16 (45.71) | 16 (80) | 4 (20) | 7 (70) | 3 (30) | 0.147 c |
| Ceftriaxone | 16 (45.71) | 19 (54.29) | 17 (85) | 3 (15) | 6 (60) | 4 (40) | 0.017 c |
| Imipenem | 20 (57.14) | 15 (42.86) | 15 (75) | 5 (25) | 7 (70) | 3 (30) | 0.388 b |
| Nitrofurantoin | 13 (37.14) | 22 (62.86) | 13 (65) | 7 (35) | 5 (50) | 5 (50) | 0.136 c |
a Values are expressed as No. (%).
b Fisher’s exact test
c Chi-squared test
4.4. Detection of Antibiotic Resistance Genes
Resistance genes were searched to understand the phenotypic data related to strain resistance (Table 6). This table showed that 60% of the strains that developed resistance to antibiotics such as norfloxacin, nalidixic, and ciprofloxacin, as the antibiotics of the quinolone and fluoroquinolone family, possessed the resistance genes qnrA, qnrB, qnrS with the characteristic of quinolones (P < 0.05). Similarly, above 60% of the strains resistant to ampicillin, cephalothin, and imipenem had resistance genes belonging to the beta-lactamase family, including blaSHV and blaCTX-M. Thus, these results show that the different resistance genes present in the genomes of E. cloacae strains were expressed. These strains are multi-resistant, and these resistances already reach the carbapenem class. This requires important surveillance.
| Antibiotic/ Gene | qnrA | qnrB | qnrS | tet A | tet B | sul1 | ant (2)Ia | ant (3)Ia | aac(3)IIa | blaSHV | Bla CTX-M |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NA; n = 45; (P-value = 0.000*) | 27 | 9 | 5 | ||||||||
| 60 | 20 | 11.11 | |||||||||
| CP; n = 30; (P-value = 0.000*) | 20 | 8 | 2 | ||||||||
| 66.67 | 26.67 | 6.67 | |||||||||
| NOR; n = 25; (P-value = 0.000*) | 15 | 6 | 1 | ||||||||
| 60 | 24 | 4 | |||||||||
| TE; n = 48; (P-value = 0.275*) | 35 | 30 | |||||||||
| 72.92 | 62.5 | ||||||||||
| SXT; n = 55 | 40 | ||||||||||
| 72.72 | |||||||||||
| K; n = 40; (P-value = 0.822*) | 18 | 17 | |||||||||
| 45 | 42.5 | ||||||||||
| AN; n = 45; (P-value = 0.67*) | 20 | 18 | |||||||||
| 44.44 | 40 | ||||||||||
| GM; n = 35 | 15 | ||||||||||
| 42.86 | |||||||||||
| AM; n = 50; (P-value = 0.309*) | 32 | 27 | |||||||||
| 64 | 54 | ||||||||||
| CF; n = 20; (P-value = 0151) | 16 | 19 | |||||||||
| 80 | 95 | ||||||||||
| IMP; n = 20; (P-value = 0.342*) | 12 | 9 | |||||||||
| 60 | 45 |
5. Discussion
The present study aimed to specify the molecular characterization of E. cloacae strains involved in UTIs. Out of the 65 E. cloacae strain isolates, above half of the strains were resistant to several classes of antibiotics. This reflects the bacterial multi-resistance characterizing the strains involved in UTIs (24). The bacterial multi-resistanceinclude aminoglycosides, tetracyclines, quinolones, penicillin, macrolides, and sulfonamides. The management processes are thus limited (25). Thus, this constitutes a break from the cure by antibiotic therapy. The resistance to beta-lactam antibiotics, especially carbapenems, shows that the antibiotics of last resort are minimal. In the case of UTIs with multidrug-resistant bacteria, physicians will be faced with a significant lack of solutions in a short period.
This issue shows the real public health problem with the antibiotic resistance of uropathogenic bacteria. This confirms the finding of earlier studies by Hryniewicz et al. (11) on the increasing extent of resistance among pathogens in UTIs to conventional drugs. A few years ago, during epidemiological surveillance, there was a significant decrease in the antibiotic susceptibility of strains involved in UTIs. These observations would be due to the acquisition and transmission of resistance genes between bacteria through plasmids by conjugation or transformation. Self-medication and non-compliance with dosage would also be factors, which could have considerably increased resistance in E. cloacae strains. However, in contrast to the findings of our study, in which the sensitivity to imipenem of the strains was only 20%, the sensitivity was above 80% in their study (12). According to Matsumoto and Muratani (26), imipenem has high antimicrobial activity against fresh urinary isolates such as E. cloacae. This strain is and remains an important opportunistic and multi-resistant pathogen for humans, which can create the epidemics of nosocomial infections (16). Nevertheless, the minimum inhibitory concentrations of different antibiotics tested should have been determined.
All isolated E. cloacae strains were biofilm producers, and above half of the isolates were strongly associated with biofilm production. Moreover, these findings corroborate with those reported by Sabir et al. (27). They also found that E. cloacae species had the highest biofilm production (87.5%) among pathogens isolated from catheter-associated UTIs with bacterial biofilm. Biofilm formation is one important characteristic of E. cloacae and other Enterobacteriaceae, which has been used in the rapid bioremoval of hydrocarbons in diesel fuel. In other words, the presence of this species in the urinary tract is a real threat to the patient. This study demonstrated the predominant role of biofilm production in increasing the resistance trait developed by E. cloacae. It showed that biofilm production increases antibiotic resistance in E. cloacae isolates.
Moreover, this finding is in line with the findings of Sabir et al. and Maheshwari et al. (27, 28). Sabir et al. (27) showed that biofilm-producing strains were highly resistant to antibiotics due to their slow penetration, a resistant phenotype, and an altered microenvironment. Maheshwari et al. (28) also documented that the presence of biofilm was a factor affecting the several-fold increase of antibiotic resistance. The biofilm formation represents a means of protection for E. cloacae and defense against antibiotics.
The different resistance genes were expressed in the genomes of E. cloacae strains isolated from our samples. The bla SHV genes were more frequent (64%, 80%, 60%) than the bla CTX-M genes (54%, 95%, 45%). Furthermore, this result does not confirm the findings of Cabral et al. (29), who claimed that the CTX-M genes were most expressed. According to a study conducted in Brazil in 2017, the bla CTX-M was more frequent in isolates from infection sites (16). As the phenotypic expression is only the result of the composition of the genetic material, the PCR results can be simply understood. Furthermore, no relationship was detected between quinolone resistance genes and the presence of beta-lactam genes. Azargun et al. (30) reported a significant association between the prevalence of plasmid resistance to quinolones and ESBL-producing isolates. No carbapenem resistance gene (NDM, VIM, OXA-48) was searched, while the susceptibility test considered carbapenem class antibiotics. This means that this study was limited in terms of molecular characterization. Since we are witnessing the emergence of the mcr-1 positive strains of Enterobacteriaceae worldwide, this gene could have been searched to study its epidemiology (31).
5.1. Conclusions
UTIs are a genuine public health concern due to the involvement of multi-resistant strains such as E. cloacae. The potentials to produce biofilm and antibiotic resistance genes are essential elements giving this strain a multi-resistant and defensive strength. Accordingly, the management of these infections is difficult since antibiotic therapy is severely limited.