1. Background
During 2019 - 2021, the world faced an unprecedented challenge in modern history. The SARS-CoV-2 pandemic, seemingly started in November 2019 in Wuhan, China, caused infections in over 370 million people worldwide, accounting for 5.6 million deaths (1). At the moment, it is impossible to estimate the total social and economic costs of limiting the spread of coronavirus. We will probably never know the total number of sufferers of the 21st century pandemic, which significantly affected healthcare in Poland, especially in the late 2020 and the early 2021. Clostridium perfringens is a spore-forming bacterium producing multiple toxins, primarily responsible for numerous disorders such as gastrointestinal disturbance, poor wound healing (etiology of gas gangrene), and systemic symptoms (2). This bacterium has also been described as an etiologic factor of antibiotic-associated diarrhea (AAD) (3). All major toxins produced by C. perfringens have cytotoxic effects.
The data concerning C. perfringens food poisoning in Poland presented in the epidemiological reports of the National Institute of Hygiene are 19 in 2021, six in 2020, and one in 2019 (4, 5). According to the reports, in 2021, there has been an abrupt increase in the incidence of food poisonings caused by C. perfringens. Similar to C. difficile spores, C. perfringens spores also could be present in the hospital environment. The statistics on the prevalence of CDI in Poland presented in the epidemiological reports of the National Institute of Hygiene is alarming. The incidence rate of CDI was 10.139 in 2020, with the morbidity rate of 26.44 per 100.000 people. In 2021, there was a remarkable growth in the incidence of CDI with 21.149 confirmed cases with a morbidity rate of 55.14 of 100.000 people (4, 5). We hypothesized that the infection prevention and control measures implemented in the urology hospitals to prevent COVID-19 transmission, including transmission inside the hospital wards (H2O2 fogging), educational programs, increased focus on hand hygiene compliance, and others, would also affect HA-CDI.
Our previous paper revealed the increasing prevalence of urosepsis associated with extended spectrum beta-lactamase (ESBL)-producing Gram-negative Enterobacteriaceae in this urology center during 2017 - 2020, highlighting the importance of infection monitoring (6). To this end, the present study aimed to describe the impact of sanitary and epidemiological measures adopted for preventing the spread of SARS-CoV-2 infection on the occurrence of Clostridium spp. spores in this hospital environment. The SARS-CoV-2 pandemic changed the routine medical treatment schemes. Due to the lack of unambiguous recommendations regarding the treatment of patients infected with the virus at the beginning of the COVID-19 pandemic, the use of antibiotics (aimed at combating accompanying infections) and proton-pump inhibitors (PPIs) aimed at preventing the onset of stress ulcer, has increased.
Despite unflagging interest in the SARS-CoV-2 pandemic, infections caused by pathogens such as C. difficile and C. perfringens have also attracted researchers’ attention. These pathogens can cause a broad spectrum of symptoms, ranging from mild abdominal pain by diarrhea (C. perfringens) (2) to life-threatening pseudomembranous colitis (C. difficile) (7). Both pathogens have also been characterized as the etiological agents of AAD (3, 8). Regarding the importance of the easy spread of the above-mentioned pathogens in the hospital environment, this bacterium can produce spores, extremely resistant to the actions of physical and chemical factors, which are often used in the sanitation process of hospital rooms (9). Moreover, when checking the cleanliness of these rooms, media capable of detecting spores are not routinely used, and only media capable of detecting active forms of pathogens, especially aerobic bacteria, are frequently utilized.
2. Objectives
Our previous paper (10) described the possibility of culturing C. difficile and other Clostridium spp. from the hospital environment using C diff Banana Broth™ (Hardy Diagnostics, Santa Maria, USA). Accordingly, this study aimed to detect the Clostridium spp. spores using C diff Banana Broth™ in the environment of the urology center and evaluate the antibiotic susceptibility and toxin profile of the isolated strains.
3. Methods
3.1. Samples and Media
The study was conducted in November 2020 in an 86-bed urology hospital in Silesia, Southern Poland, a few weeks after detecting numerous cases of SARS-CoV-2 infection among medical staff. This event resulted in the closure of the hospital for two weeks and the adoption of sanitary measures (hydrogen peroxide fogging). Some data were also collected from the hospital pharmacy on the consumption of soap, disinfectants, proton pump inhibitors (PPIs), and antibiotics in 2019, 2020, and 2021. The samples were taken from rooms where CDI had been confirmed in patients during the last year. Sterile disposable swabs previously moistened with PBS were used to collect environmental samples. To this end, 58 samples were collected and inoculated using the C diff Banana Broth™. The swabs were taken from a variety of similar-sized surfaces (10 × 10 cm template was used) and evaluated by investigators collecting the samples. The C diff Banana Broth™ is designed to allow spores to transform into a vegetative form. It does not require anaerobic conditions for incubation. Initially, the medium is red and then turns yellow after spore germination (11). This is due to the fermentation of mannitol added instead of glucose in order not to have a repressive effect on the cytotoxicity of C. difficile. The color change can be simply distinguished.
3.2. Isolation and Identification of Clostridium spp.
Fifty-eight samples were collected from different places, especially from places difficult to clean. The C diff Banana Broths™ with positive and negative controls were incubated for two weeks at 37°C under aerobic conditions, controlling their limpidity and color per 24 hours. Yellow broths (positive results) were inoculated on selective media for C. difficile (CLO and CDIFF bioMérieux, Marcy L’Etoile, France) and Columbia Blood Agar (CBA) and cultured for 48 h anaerobically at 37°C (Whitley A35 Workstation, UK). The growing colonies were identified with the VITEK 2 Compact (bioMérieux, Marcy L’Etoile, France), evaluated for hemolysis (double), tested for antibiotic susceptibility, and examined for toxin genes.
3.3. Multiplex PCR Performing
The grown singular colonies were subcultured in the BHI broth used for the DNA isolation. The GeneMATRIX DNA Purification Kit by DNA Gdansk, PL, was used for the same purpose. The genes encoding A, B, and binary toxins in C. difficile were detected by multiplex PCR (mPCR), as previously described (12). Moreover, mPCR was used to detect the presence of toxin alpha-cpa, toxin beta-cbp, enterotoxin-cpe, iota toxin-cpiA, and epsilon toxin-etxin C. perfringens, as described by Meer and Songer (13). The strains of C. difficile VPI 10463, C. difficile ATCC700057, C. difficile CCUG 20309, and C. perfringens ATCC 1324 were used as quality controls in mPCR. The negative control was the sterile BHI broth. The reaction condition was as follows: 120 s at 94°C as initial denaturation, followed by 35 cycles of 60 s at 94°C for denaturation, 60 s at 53°C as annealing, 60 s at 72°C for extension and final extension at 72°C for 10 min. The cpb2 gene was detected according to Garmory et al.’s study (14) with the following reaction condition: 120 s at 92°C as initial denaturation, followed by 35 cycles of 60 s at 92°C for denaturation, 60 s at 53°C as annealing, 60 s at 72°C for extension and final extension at 72°C for 10 min. The amplification was performed using Eppendorf Mastercycler ep. Table 1 presents the primers and reaction conditions. Electrophoresis was performed on an agarose gel stained with ethidium bromide (120 min, 110 V). The result was imaged by the Syngene G: BOX.
| Gene | F sequence | R sequence | Reaction Conditions | Product size (bp) | References |
|---|---|---|---|---|---|
| cpa | GCTAATGTTACTGCCGTTGACC | TCTGATACATCGTGTAAG | 94°C 2 min, 94°C 1 min, 53°C 1 min, 72°C 1 min, 72°C 10 min; 35 cycles | 324 | (13) |
| cpb | GCGAATATGCTGAATCATCTA | GCAGGAACATTAGTATATCTTC | 196 | (13) | |
| cpe | GGAGATGGTTGGATATTAGG | GGACCAGCAGTTGTAGATA | 233 | (13) | |
| cpiA | ACTACTCTCAGACAAGACAG | CTTTCCTTCTATTACTATACG | 446 | (13) | |
| etx | TGGGAACTTCGATACAAGCA | GGACCAGCAGTTGTAGATA | 655 | (13) | |
| cpb2 | GCGAATATGCTGAATCATCTA | GCAGGAACATTAGTATATCTTC | 92°C 2 min, 92°C 1 min, 53°C 1 min, 72°C 1 min, 72°C 10 min; 35 cycles | 567 | (14) |
aClostridium perfringens toxin genes: Alpha, cpa; beta, cbp; enterotoxin, cpe; iota, cpiA; epsilon, etx; and beta 2 toxin, cpb2
3.4. Antibiotic Susceptibility Testing of Isolates
E-tests (namely bioMérieux, Marcy L’Etoile, and France) were used to assess antibiotic susceptibility for nineantibiotics: Metronidazole (range 0.0094 - 2 μg/mL), vancomycin (0.023 - 1 μg/mL), erythromycin (0.38 - 4 μg/mL), clindamycin (0.016 - 12 μg/mL), moxifloxacin (0.032 - 0.5 μg/mL), rifampicin (0.002 – 0.004 μg/mL), piperacillin with tazobactam (0.023 - 4 μg/mL), benzylpenicillin (0.016 – 0.5 μg/mL), imipenem (0.016 - 32 μg/mL). Brucella Blood Agar plates with vitamin K and hemin and Schaedler Broth with vit K3 (bioMérieux, Marcy L’Etoile, France) were used to determine the minimum inhibitory concentration (MIC) values. The plates were incubated for 48 h at 37°C anaerobically according to the manufacturing instruction.
The susceptibility of the strains was interpreted according to the EUCAST (European Committee on Antimicrobial Susceptibility Testing, Version 10.0, valid 2020-01-01mk) recommendations (15). We used EUCAST from 2004 when Poland joined the European Union. For antibiotic susceptibility testing, we choose antibiotics inducing CDI and using for the treatment of Clostridium spp. infection. Moreover, clindamycin, moxifloxacin, piperacillin/tazobactam, penicillin, and imipenem are often used in the urology department. Interpretations for Gram (+) anaerobes and C. difficile were used, and MIC > 256 μg/mL was considered resistant to erythromycin. The strains of C. difficile VPI 10463, C. perfringens ATCC1324, Bacteroides thetaiotaomicron ATCC 29741, and B. fragilis ATCC 25285 were used as quality control strains. All control results were within acceptable limits.
4. Results
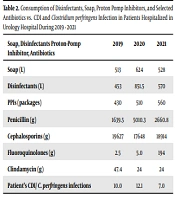
The effect of the SARS-CoV-2 pandemic on hospital treatment cannot be underestimated. This applies both to changes in the consumption of basic drugs used for the treatment of viral infection and the consumption of hygiene products and personal protection measures. The hospital data demonstrated a significant increase in the consumption of disinfectants: Their consumption increased by 187.97% in 2020 compared to 2019 and by 125.83% in 2021 compared to 2019 (Table 2). The soap consumption in 2021 increased by 102.92% compared to 2019. In 2020, there was a notable increase in soap consumption by 121.63% compared to 2019 (Table 2). The increased consumption of selected antibiotics (namely penicillin and fluoroquinolones) was also observed. Although the consumption level of cephalosporines was about the same, the clindamycin consumption decreased. The consumption of PPIs also increased during 2019, 2020, and 2021.
| Soap, Disinfectants Proton-Pump Inhibitor, Antibiotics | 2019 | 2020 | 2021 |
|---|---|---|---|
| Soap (L) | 513 | 624 | 528 |
| Disinfectants (L) | 453 | 851.5 | 570 |
| PPIs (packages) | 430 | 510 | 560 |
| Penicillin (g) | 1639.5 | 5010.3 | 2660.8 |
| Cephalosporins (g) | 19627 | 17648 | 18914 |
| Fluoroquinolones (g) | 2.5 | 5.0 | 194 |
| Clindamycin (g) | 47.4 | 24 | 24 |
| Patient’s CDI/ C. perfringens infections | 10/0 | 12/1 | 7/0 |
In 2021, CDI was confirmed by the presence of toxins in stools in seven samples from 14 suspected patients (7/14). In 2020 and 2019, there were 12/27, and 10/27 confirmed CDI cases, respectively. Interestingly, one case of C. perfringens infection (wound isolate) was confirmed in 2020. Table 2 presents some information about the consumption of soap, disinfectants, selected antibiotics, PPIs, C. perfringens, and CDI incidences. In general, the following 16 Clostridium spp. strains were cultured from the 58 collected swabs: 11 strains of C. perfringens, two strains of C. baratii, and one each of C. paraputrificum, C. difficile, and C. clostridioforme (Table 3). The eleven strains of C. perfringens possessed the cpa toxin gene, 7 out of 11 strains also demonstrated the cpb2 gene, two strains possessed the cpiA gene, and the presence of cpb gene was confirmed in one strain.
Table 3 demonstrates the toxin genes of Clostridium spp. isolates and also the places in the hospital environment, where the strains were isolated. All cultured strains were sensitive to metronidazole, vancomycin, moxifloxacin, penicillin/tazobactam, and rifampicin. The C. difficile isolate presented the tcdA+/tcdB+/cdtA/cdtB+/ermB(-) profile and was sensitive to metronidazole, vancomycin, moxifloxacin, and rifampicin. Two out of 11 C. perfringens strains were resistant to erythromycin and clindamycin. Table 4 provides some details on the MIC50, MIC90, and the geometric means and percentage of antibiotic-resistant strains.
| Strains | Place of Swabbing | Detected Toxin genes |
|---|---|---|
| 1. C. perfringens | Patient’s room sink | cpa, cpb2 |
| 2. C. perfringens | Patient’s toilet floor | cpa, cpb2 |
| 3. C. perfringens | Patient’s toilet doorknob | cpa, cpiA |
| 4. C. perfringens | Patient’s room sink | cpa, cpb |
| 5. C. perfringens | Patient’s room sink | cpa, cpb2 |
| 6. C. perfringens | Patient’s room sink | cpa, cpb2 |
| 7. C. perfringens | Patient’s toilet doorknob | cpa, cpiA |
| 8. C. perfringens | Patient’s room bed side rail | cpa, cpb2 |
| 9. C. perfringens | Toilet for Staff floor | cpa |
| 10. C. perfringens | Floor under bookshelf | cpa, cpb2 |
| 11. C. perfringens | Floor under desk in doctor’s office | cpa, cpb2 |
| 1. C. baratii | Patient’s room sink | |
| 2. C. baratii | Patient’s room bed side rail | |
| C. difficile | Patient’s room bed side rail | tcdA/tcdB; cdtA/cdtB;ermB (-) |
| C. clostridioforme | Patient’s room toilet floor | |
| C. paraputrificum | Patient’s room drawer handle |
| Antibiotics | MIC50 (μg/mL) | MIC90 (μg/mL) | GM | Resistant Strains (% EUCAST) | Resistant EUCAST (μg/mL) |
|---|---|---|---|---|---|
| Metronidazole | 1 | 1 | 0.770 | 0 | > 4 |
| Vancomycin | 0.38 | 0.75 | 0.419 | 0 | > 2 |
| Erythromycin a | 3 | 256 | 4.854 | 18.2 | IE a |
| Clindamycin | 0.064 | 8 | 0.342 | 18.2 | > 4 |
| Moxifloxacin | 0.25 | 0.38 | 0.241 | 0 | > 4 b |
| Rifampicin | 0.003 | 0.003 | 0.003 | 0 | > 0.004 b |
| Piperacillin/Tazobactam | 0.047 | 0.064 | 0.049 | 0 | > 16 |
| Penicillin | 0.064 | 0.064 | 0.048 | 0 | > 0.5 |
| Imipenem | 0.094 | 0.125 | 0.097 | 0 | > 2 |
a No resistant values. Values > 256 μg/mL was assumed as a resistant strain.
b No MIC value, ECOFF value was assumed for the study purposes.
5. Discussion
The SARS-CoV-2 pandemic has completely changed human habits. The implemented recommendations increased the use of personal protective equipment and disinfectants worldwide (16-18). In a survey in China, it was estimated that about 65% of the respondents had never disinfected their hands before the pandemic (16). Moreover, 17.6 % of the respondents stated that disinfection products being used for home environment have increased at least ten times (16). Nowadays, there are reports of a change in the microbiome caused by the increased usage of disinfectants and sanitizers during the pandemic (19). This especially comes true for the increasing resistance of Enterococcus faecalis (19, 20). Because of the remarkably increased usage of disinfectants (21) included in this study, it is suggested to use sporicides to disinfect hospital surfaces and floors.
Many studies have documented the effect of the SARS-CoV-2 pandemic on CDI and the changes in gut microbiota. Many theories have also explained this phenomenon. Ponce-Alonso et al. referred to the decreased patient mobility during the COVID-19 pandemic (22). Furthermore, the increased use of antibiotics was observed in their study, with third-generation cephalosporins and macrolide being the most commonly chosen ones (22). In an analysis by Luo et al., a decrease in C. difficile tests was reported during the pandemic compared to the pre-pandemic era (23). This might have been associated with gastrointestinal symptoms such as diarrhea, attributed to SARS-CoV-2. At the same time, the incidence rate of CDI did not decrease (23). A study in Detroit by Sandhu et al. described an increase in the CDI incidence during March-April 2020, compared to their incidence during January-February 2020 (24). In this study, Khanna and Kraft concluded that hospitalized COVID-19 patients were at risk for developing CDI. Furthermore, the co-infection of CDI and SARS-CoV-2 may be associated with a higher complication rate (25).
Lewandowski et al. presented an alarming picture of CDI in the era of SARS-CoV-2 (26). There was a significant increase in CDI during the COVID-19 pandemic (10.9%) compared to the pre-pandemic period (2.6%). The underdiagnosis of patients and the inappropriate use of antibiotics are two main unsolved issues, which were exacerbated during the SARS-CoV-2 pandemic. Moreover, the consequences of the direct action of the virus on the host (the damage of intestinal mucosa and alteration of gut microbiota) affected the CDI development in patients with SARS-CoV-2 (26). During the further waves of the pandemic, CDI prevention, control, and management must play more critical roles in the healthcare responses, especially for elderly patients. The SARS-CoV-2 pandemic caused difficulties in patient care, not having been observed earlier in modern medical history. Empirically, both antibiotics and PPIs were used by patients. Moreover, the consumption of alcohol-based disinfectants has increased significantly (16-19). These factors are documented as the risk factors for CDI development and C. perfringens infection (7, 27).
We believe that the increased usage of antibiotics, alcohol-based disinfectants, and other medication during the COVID-19 pandemic (proton pump inhibitors), as the main risk factors of CDI development, increased the prevalence of spores in our hospital. Alcohol-based disinfectants have also been described as the cause of disturbed microbiome in the concerned environment. In their study, Durovic et al. stated that among the C. difficile transmission pathways, contact with symptomatic carriers (53.3%), the hospital environment (40.0%), and asymptomatic carriers (20%) play the most critical roles (28). Janezic et al. concluded that shoes’ soles had high C. difficile contamination rates (43%) and were included in the CDI control management (29, 30). Patients hospitalized in the urology hospital are also burdened with the diseases of the urinary or genitourinary system, requiring surgical treatment or a procedure to permanently maintain catheters in the urinary system (indwelling catheters, DJ catheters, nephrostomies). Those patients commonly require antibiotic therapy due to the increased risk of urinary tract infection. A large population of the patients had oncological problems or required renal replacement therapies.
The effectiveness of the disinfection process may be evaluated in different ways (31). However, none of the commonly used methods provides more information about spores in the hospital environment. Accordingly, the present study aimed to estimate the prevalence of spores using C diff Banana Broth™ enabling spore germination. The prevalence of positive Clostridium spp. samples was 16 out of 58 (27.6%). Moreover, our previous study in another hospital confirms a similar percentage of positive cases (10). In Pittsburgh (32), the reported frequencies were larger, with 50% for positive floor swabs and 0% for positive bed swabs. It is worth noting that in our study, only 3.16% of the strains were cultured from floor swabs.
The inconsistency may be due to the slightly different methods adopted for collecting the swabs in these two studies. In an American survey, the researchers used one Banana Broth tube per room to swab all surfaces and another one to collect the floor swabs. They reported that their findings might have been affected by different agents used for disinfecting surfaces (sporicidal agent) and floors (non-sporicidal agent) (32). Their study also indicates the necessity of using sporicidal agents to disinfect the floors in the case of increased CDI incidence. They also concluded that C diff Banana Broth™ could greatly contribute to the CDI prevention efforts.
Although C diff Banana Broth™ is devoted to detecting C. difficile spores, we have managed to get the germination of spores other than C. difficile by providing prolonged germination time. Among the cultured strains, apart from C. difficile, we have obtained 11 C. perfringens strains, two strains of C. baratii, and one each of C. paraputrificum and C. clostridioforme. Despite a lower potential for sporulation, a high frequency of C. perfringens spores was observed. Eleven isolates possessed the cpa gene, among which seven isolates had an additionally cpb2 gene. In this regard, cpa has been proved as the main malignancy factor in infection caused by C. perfringens. In the affected tissues, the toxin alpha causes both myonecrosis and gas gangrene.
The presence of beta2 toxin was described in the context of necrotizing enterocolitis and showed higher frequency in animals suffering from enteritis and infectious diarrhea. Although the cpb2 gene was confirmed in the pathogenicity of food poisoning, limited data exists on its pathogenicity in humans. All cultured strains were sensitive to metronidazole, vancomycin, moxifloxacin, penicillin/tazobactam, and rifampicin. Although the literature describes metronidazole-resistant C. difficile strains, we failed to confirm this finding (33). No strain of C. perfringens was resistant to benzylpenicillin G, the antibiotic of the first treatment option for C. perfringens infections (15). The resistance to clindamycin in our isolates is alarming and exceeds the values reported in the literature (34).
Our study has several limitations. It is a single-center analysis, and further investigations can improve the findings. Moreover, it focuses on the urological hospital environment during the COVID-19 pandemic after H2O2 fogging; thus, future researchers should confirm the findings in other hospitals during longer observations under standardized conditions. To sum up, further studies in other urology centers and medical wards, during longer periods, with larger sample sizes are recommended.
5.1. Conclusions
Regardless of the performed H2O2 fogging, the antibiotic-resistant, toxigenic strains of C. perfringens (69%) from the urology hospital environment were cultured using C diff Banana Broth™, highlighting the need to develop necessary sanitary and epidemiological procedures in this hospital.