1. Background
Yeasts and yeast-like fungi are single-celled opportunistic organisms that reproduce through budding. Generally, yeasts are incapable of causing disease in healthy human and animal hosts; for that, they require a substantial break in the host's defense system (1). Candida species are the most common yeasts that cause fungal infections, especially urinary tract infections, and can cause candiduria (2). Candida species seen in the urine of immunocompromised people may indicate candiduria development. The prevalence of candiduria has risen considerably in the previous two decades in patients hospitalized in nephrology and intensive care units (ICUs) (3).
Broad-spectrum antibiotic therapies, urinary catheters, intravascular catheters, urinary tract disorders, immunosuppressive therapies, radiation, immunosuppressive agents, prolonged hospitalization, diabetes mellitus, and malignancies are considered predisposing factors for developing candiduria in hospitalized patients (2, 3). Studies showed that despite repeated blood cultures, half of the patients with positive candidiasis in autopsy specimens did not exhibit any symptoms in their lifetime. In contrast, most were positive for candidiasis in urine samples (2, 3). Early diagnosis of the etiology of candiduria is challenging, which might cause therapeutic delays. As a result, antifungal agents (such as fluconazole) are commonly used to avoid invasive fungal infections in immunosuppressed and immunocompromised patients (4).
Although Candida albicans is the most common cause of candiduria, non-albicans Candida species, particularly C. glabrata, and C. krusei, can be involved in the development of invasive candidiasis (1). Specific identification of Candida species helps choose the appropriate treatment because several non-albicans Candida species, such as C. krusei, are intrinsically resistant or less susceptible to several classes of antifungals, whereas others, including C. glabrata, develop acquired resistance following exposure to antifungal agents. Also, resistance to amphotericin B has been observed in C. lusitaniae, C. guilliermondii, C. kefir, and C. rugosa. On the other hand, susceptibility to novel antifungals such as echinocandins, including Anidulafungin, has been reduced in C. guilliermondii and C. parapsilosis (5).
2. Objectives
The current investigation attempted to evaluate the frequency and species distribution of fungal isolates causing candiduria among patients hospitalized in the nephrology and intensive care unit wards of two hospitals (Bu Ali and Labbafinezhad) in Tehran, Iran, as well as determines the antifungal susceptibility patterns of isolated species. Due to the different dispersion and drug susceptibility patterns of Candida species at different time intervals, this study will provide essential information for researchers and medical staff and pave the way for further studies.
3. Methods
3.1. Study Participants, Sample Collection, and Clinical Information
This descriptive cross-sectional research was carried out over six months, from September 2019 to February 2020, on 104 patients admitted to the nephrology and ICU wards of Bu Ali and Labbafinezhad hospitals in Tehran, Iran. The study population included patients with urinary tract infections, diabetic patients, people with digestive disorders, having a urinary catheter for a long time and not responding to some antifungal drugs, and those suspected of candidiasis, admitted to nephrology departments and ICU wards of Bu Ali and Labbafinezhad hospitals in Tehran. After identifying individuals who met the inclusion criteria, their demographic and clinical information such as age, gender, underlying disease, history of treatment with broad-spectrum antibiotics, and presence of urinary tract catheter was recorded.
3.2. Sample Preparation and Mycological Examinations
After preparing the sampling equipment for patients without a urinary catheter, each patient's urine sample was collected in a sterile container that had been previously labeled with the patient's information. In patients with urinary catheters, urine was collected from the upper part for 20 to 30 min after clamping the catheter. The samples were aspirated into syringes and collected into covered sterile containers with the patients’ information labeled on them, and then immediately were transferred to the laboratory for mycological examinations. The urine samples were centrifuged before direct microscopic analysis, and the sediment was used for direct examination with 10% potassium hydroxide (KOH 10%). All specimens were cultured on Sabouraud dextrose agar (SDA) with chloramphenicol and incubated for three days at 35°C. In the culture plates, the colonies obtained after the incubation period were counted, and only the specimens with a colony count of ≥ 1 × 103 were considered as positive cultures for candiduria. The Candida isolates were purified through visualization of the color of the colony on the CHROMagar Candida medium.
3.3. Molecular Techniques
3.3.1. DNA Extraction
The DNA from the fungal genome was initially isolated following the manufacturer's recommended directions with the Roche High Pure PCR Template Preparation kit (Roche, Germany).
3.3.2. PCR Analysis and Sequencing
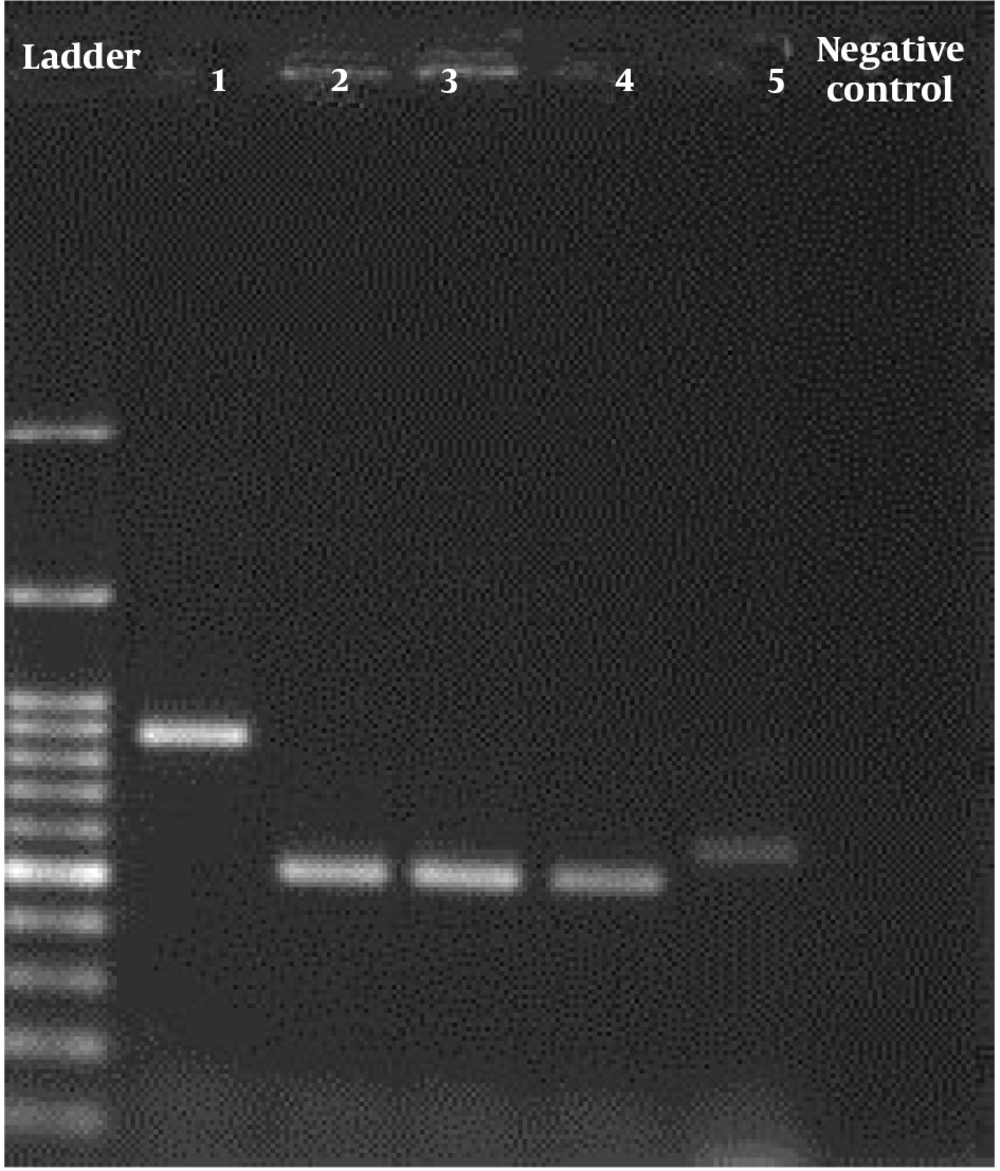
We used ITS1 (5,-TCC GTA GGT GAA CCT GCG G-3,) and ITS4 (5,-TCC TCC GCT TAT TGA TAT GC-3,) primers (Bioneer, South Korea) for replication in the following thermal conditions: 95°C for 5 minutes, then 35 cycles of 30 s at 94°C, 45°C for 30 s, and 72°C for 45 s, followed by one final extension at 72°C for 5 minutes. Staining with DNA-safe stain and electrophoresis on a 1.5 percent agarose gel were used to analyze the PCR results (Figure 1). PCR products were purified with the Silica Bead DNA Gel Extraction Kit (K0513) and were sent to Bioneer Co. for single-direction sequencing using forward primer (Bioneer, South Korea). The sequences of isolates were subjected to ClustalW pairwise alignment using the MEGA7.0.21 software and edited manually to improve the alignment accuracy and compared in the GenBank database using the BLAST. All of the sequences had been deposited in GenBank under the accession number reported in Table 1. Sequencing method was applied for confirmation of all identified fungal isolates.
| Isolate | Molecular Identification (IT’S a Gene) | GenBank Accession Number | Isolate | Molecular Identification (ITS a Gene) | GenBank Accession Number |
|---|---|---|---|---|---|
| US1b | Candida krusei | MW980765 | US14 | C. albicans | KC905069 |
| US2 | C. glabrata | MT772039 | US15 | C. glabrata | MT772072 |
| US3 | C. krusei | MT772040 | US16 | C. albicans | MT772073 |
| US4 | C. tropicalis | MT772041 | US17 | C. glabrata | MT772074 |
| US5 | C. glabrata | MT772042 | US18 | C. krusei | MT772075 |
| US6 | C. parapsilosis | EU564209 | US19 | C. glabrata | MT772076 |
| US7 | C. tropicalis | MT772043 | US20 | C. glabrata | MT772077 |
| US8 | C. krusei | MT772044 | US21 | C. albicans | MT772052 |
| US9 | C. glabrata | MT772045 | US22 | C. krusei | FJ515204 |
| US10 | C. albicans | MT772046 | US23 | C. glabrata | KU992392 |
| US11 | C. krusei | MT772061 | US24 | C. albicans | MT772079 |
| US12 | C. albicans | MT772047 | US25 | C. parapsilosis | EU564205 |
| US13 | C. glabrata | MT772048 | US26 | C. albicans | MK793223 |
Abbreviations: ITSa, internal transcribed spacer; USb, urine sample.
3.4. Antifungal Susceptibility Test
The isolates' susceptibilities to fluconazole (Sigma Chemical Co., St Louis, Mo.), amphotericin B (Bristol-Myers SP, Dublin, Ireland), caspofungin (Merck Sharp &Dohme, Whitehouse Station, NJ), and itraconazole (Janssen-Cilag, High Wycombe, UK) were determined using the standard broth microdilution method as described in the Clinical and Laboratory Standards Institute document M27-A, which is specific for testing antifungal susceptibility of yeasts (6). Candida krusei ATCC 6258 standard strain has been used as quality control (QC). After 48 hours of incubation at 35°C, the microplates were monitored to estimate the MIC of tested drugs, according to CLSI protocol M27 4th ed (6). The growth and non-growth of yeasts and standard strains in wells containing the drug were compared with the positive control well using a special mirror to determine minimum inhibitory concentrations (MICs). The susceptibility tests were interpreted according to CLSI M27 4th ed as shown in Table 2 (6).
| Candida Species | Antifungal Agents | M27 4th ed Breakpoints | ||
|---|---|---|---|---|
| S | I | R | ||
| Candida glabrata | AmB | ≤ 1 | - | ≥ 2 |
| FLZ | ≤ 16 | 32 | ≥ 64 | |
| ITC | ≤ 0.125 | 0.25 - 0.5 | ≥ 1 | |
| CASP | ≤ 0.125 | 0.25 | ≥ 0.5 | |
| C. albicans | AmB | ≤ 1 | - | ≥ 2 |
| FLZ | ≤ 16 | 32 | ≥ 64 | |
| ITC | ≤ 0.125 | 0.25 - 0.5 | ≥ 1 | |
| CASP | ≤ 0.125 | 0.25 | ≥ 1 | |
| C. tropicalis | AmB | ≤ 1 | - | ≥ 2 |
| FLZ | ≤ 2 | 4 | ≥ 8 | |
| ITC | ≤ 0.125 | 0.25 - 0.5 | ≥ 1 | |
| CASP | ≤ 0.25 | 0.5 | ≥ 1 | |
| C. parapsilosis | AmB | ≤ 1 | - | ≥ 2 |
| FLZ | ≤ 2 | 4 | ≥ 8 | |
| ITC | ≤ 0.125 | 0.25 - 0.5 | ≥ 1 | |
| CASP | ≤ 2 | 4 | ≥ 8 | |
| C. krusei | AmB | ≤ 1 | - | ≥ 2 |
| FLZ | ≤ 8 | 16 - 32 | ≥ 64 | |
| ITC | ≤ 0.125 | 0.25 - 0.5 | ≥ 1 | |
| CASP | ≤ 0.25 | 0.5 | ≥ 1 | |
Abbreviations: AmB, amphotericin B; FLZ, fluconazole; ITC, itraconazole; CASP, caspofungin; S, susceptible; I, Intermediate; R, resistant.
a (-) breakpoints not provided by CLSI document M27-A4.
3.5. Statistical Analysis
SPSS was used to conduct the statistical analysis (version 24, IBM, Chicago, IL). The chi-square test, relative percentages, and the 95 percent confidence interval were utilized. P-values less than 0.05 were considered statistically significant.
4. Results
Out of 104 included patients, 26 (25%) had positive cultures for Candida. They had a significant candiduria with a colony count of ≥ 1 × 103 colony forming units (CFU)/ mL. Among them, 9 patients (27.27%) were male, and 17 patients (23.94%) were female. The results of the statistical analysis demonstrated that there was no statistically significant association between the patient's gender and the incidence of candiduria (P = 0.72). All of the patients were older than 19, with the majority of them falling between the ages of 64 and 79 (Table 3). Also, among 26 patients with candiduria 12 patients (46%) had a history of treatment with broad-spectrum antibiotics and 14 patients (64%) had no such history, and the findings revealed that there was no statistically significant association between a positive history of broad-spectrum antibiotic therapy and candiduria (P = 0.154).
| Age Groups (y) | Gender | Total | |
|---|---|---|---|
| Female | Male | ||
| 19 - 34 | 1 (3.85) | 1 (3.85) | 2 (7.7) |
| 34 - 49 | 1 (3.85) | 1(3.85) | 2 (7.7) |
| 49 - 64 | 4 (15.38) | 2 (7.69) | 6 (23.07) |
| 64 - 79 | 6 (23.07) | 3 (11.54) | 9 (34.61) |
| 79 - 94 | 5 (19.23) | 2 (7.69) | 7 (26.92) |
| Total | 17 (65.38) | 9 (34.62) | 26 (100) |
a Values are expressed as No. (%).
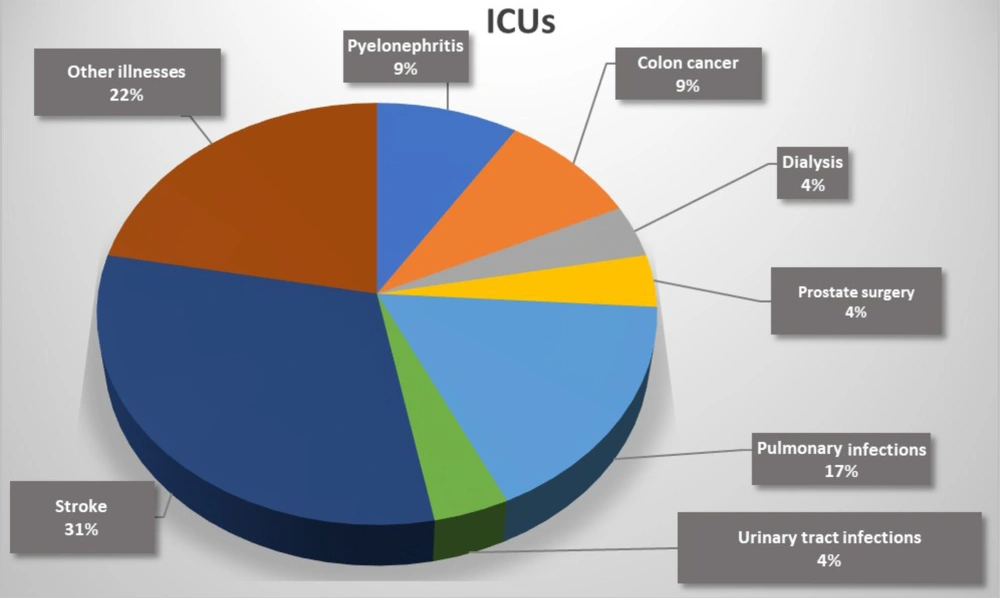
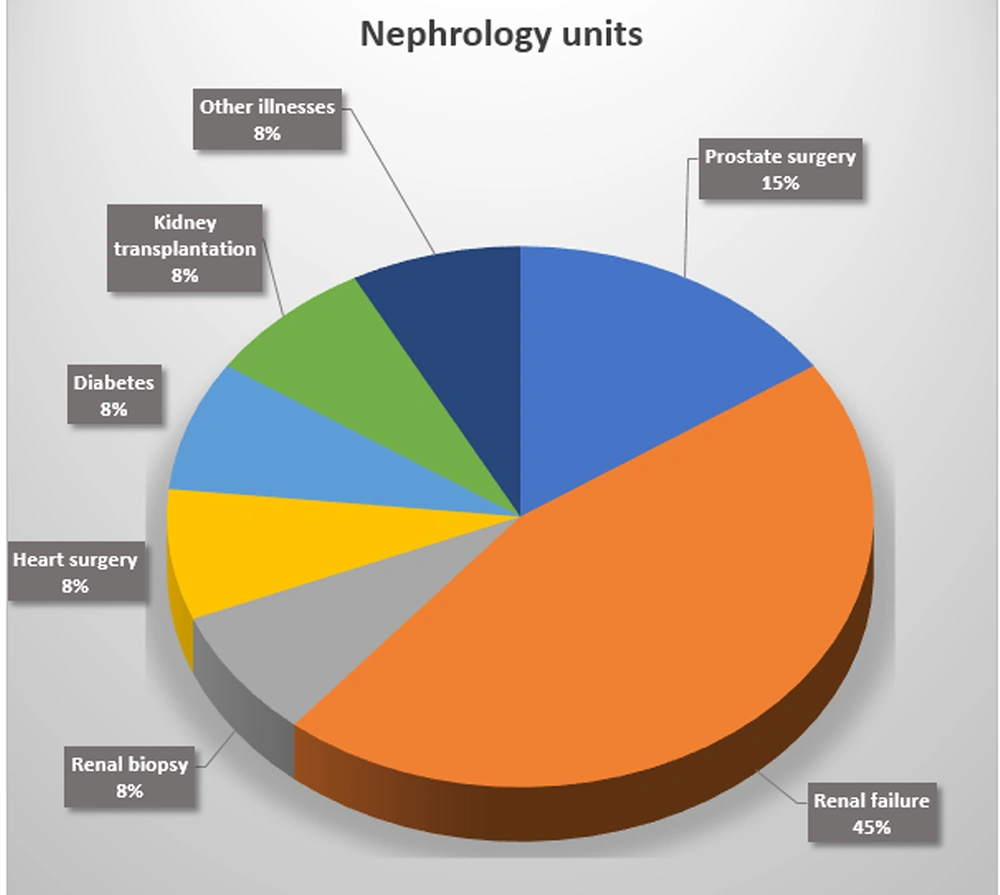
Furthermore, among 26 patients with candiduria 19 patients (73.07%) had a urinary catheter and 7 patients (26.93%) had not, and according to the chi-square test, the use of urinary catheters had a strong correlation with the incidence of candiduria (P = 0.001). The results showed that the most frequent underlying disease in patients with candiduria admitted to the ICUs was stroke (5/16, 31%) (Figure 2). Moreover, the most common underlying disease in patients with candiduria who were referred to the nephrology units was renal failure (4/10, 40%) (Figure 3). According to the results of molecular techniques, C. glabrata (n = 10, 38.64%) was the most frequent and leading cause of candiduria followed by C. albicans and C. krusei (each n = 6, 23.07%), and C. tropicalis and C. parapsilosis (each n = 2, 7.69%).
Table 4 presents detailed information regarding the results of antifungal susceptibility of the 26 Candida isolates to amphotericin B, itraconazole, caspofungin, and fluconazole. The results of the present study indicated that all tested isolates of C. albicans, C. tropicalis, C. krusei, and C. parapsilosis were susceptible to caspofungin. Also, among all isolated Candida species, one (3.8 %) isolate of C. glabrata was resistant to caspofungin and one (3.8 %) isolate of this species was resistant to amphotericin B. Generally, Candida species were highly susceptible to caspofungin (MIC range: 0.0625 - 1 µg/mL), and amphotericin B (MIC range: 0.0625 - 2 µg/mL) whereas fluconazole (MIC range: 0.25 - 64 µg/mL), and itraconazole (MIC range: 0.0625 - 16 µg/mL) showed low antifungal activity against these fungal species.
| Species (N = 26) / Antifungal Drugs | MIC Values (µg/mL) | S | I | R | Range | Mean ± SD | GM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0625 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | |||||||
| Candida glabrata (n = 10) | |||||||||||||||||
| FLZ | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 9 (90) | - | 1 (10) | 0.25 - 64 | 11.75 ± 19.41 | 3.175 | |||
| AmB | 1 | 8 | 1 | 9 (90) | - | 1 (10) | 0.5 - 2 | 1.45 ± 0.57 | 1.32 | ||||||||
| ITC | 6 | 2 | 2 | 8 (80) | 2 (20) | - | 0.0625 - 0.5 | 0.16 ± 0.17 | 0.11 | ||||||||
| CASP | 1 | 2 | 2 | 4 | 1 | 3 (30) | 6 (60) | 1 (10) | 0.0625 - 1 | 0.43 ± 0.32 | 0.30 | ||||||
| C. albicans (n = 6) | |||||||||||||||||
| FLZ | 2 | 2 | 2 | 6 (100) | - | - | 0. 25 - 2 | 0.92 ± 0.77 | 0.63 | ||||||||
| AmB | 3 | 3 | 6 (100) | - | - | 0.625 - 0.125 | 0.93 ± 0.03 | 0.08 | |||||||||
| ITC | 2 | 4 | - | - | 6 (100) | 2 - 4 | 3.33 ± 0.94 | 3.17 | |||||||||
| CASP | 1 | 5 | 6 (100) | - | - | 0.0625 - 0.125 | 0.135 ± 0.05 | 0.12 | |||||||||
| C. krusei (n = 6) | |||||||||||||||||
| FLZ | 1 | 1 | 1 | 1 | 1 | 1 | 4 (66.6) | 1 (16.7) | 1 (16.7) | 0.5 - 64 | 20.37 ± 26 | 4.75 | |||||
| AmB | 2 | 1 | 1 | 2 | 6 (100) | - | - | 0.0625 - 1 | 0.45 ± 0.41 | 0.25 | |||||||
| ITC | 1 | 1 | 4 | 1 | - | 5 | 0.125 - 4 | 3.02 ± 1.48 | 2.00 | ||||||||
| CASP | 1 | 1 | 4 | 1 | 5 | - | 0.125 - 0.5 | 0.45 ± 0.093 | 0.44 | ||||||||
| C. tropicalis (n = 2) | |||||||||||||||||
| FLZ | 1 | 1 | 1 (50) | 1 (50) | - | 1 - 4 | 2.5 ± 1.5 | 2 | |||||||||
| AmB | 1 | 1 | 2 (100) | - | - | 0.0625 - 0.125 | 0.093 ± 0.031 | 0.08 | |||||||||
| ITC | 2 | - | - | 2 (100) | 4 | 4 ± 0 | 4.00 | ||||||||||
| CASP | 1 | 1 | 1 (50) | 1 (50) | - | 0.125 - 0.5 | 0.312 ± 0.187 | 0.25 | |||||||||
| C. parapsilosis (n = 2) | |||||||||||||||||
| FLZ | 1 | 1 | 1 (50) | 1 (50) | - | 1 - 4 | 2.5 ± 1.5 | 2 | |||||||||
| AmB | 2 | 2 (100) | - | - | 0.0625 | 0.047 ± 0.015 | 0.0450 | ||||||||||
| ITC | 1 | 1 | - | - | 2 (100) | 4 - 16 | 10 ± 6 | 8 | |||||||||
| CASP | 1 | 1 | 2 (100) | - | - | 0.5 - 1 | 0.625 ± 0.37 | 0.5 | |||||||||
Abbreviations: FLZ, fluconazole; AmB, amphotericin B; Itra, itraconazole; CASP, caspofungin; S, susceptible; I, intermediate; R, resistant; SD, standard deviation; GM, geometric mean.
a All the continuous data were represented by the mean +_ SD (for normally distributed data) or by the median and interquartile (for non-normally distributed data). Categorical data were presented by the No. (%).
5. Discussion
Candiduria is one of the most common infections in hospitalized patients (2-4). In the current study, the prevalence of candiduria was estimated to be 25%. It was different from the results of studies conducted by Ghiasian et al. (0.6%) (7), Gabardi et al. (0.48%) (8), Safdar et al. (0.62%) (9), and Gholamipour et al. (3.4%) (10). This divergence in the prevalence of candiduria in different studies can be due to several factors, including climate conditions, health and economic situations, study type and population, and study length. Although according to most studies, candiduria is more common in women and is usually associated with vaginal candidiasis (2-4), in this research, there was no statistically significant difference in the incidence of candiduria between men and women. The absence of vaginal candidiasis could be an explanation for this finding (11).
According to the findings of this study, age plays a critical role in the development of candiduria, with those over 64 years old being more susceptible. Older adults are more susceptible to infections as a result of predisposing factors such as diabetes, renal failure, and arthritis. Immunosenescence occurs as people age, which implies that the immune system is attenuated. Elderly individuals can be more susceptible to infections due to a combination of the predisposing factors and the attenuation of the immune system (12). In this study, urinary tract catheterization was found to be directly linked to the development of candiduria. According to the findings of a study conducted by Jain et al, patients who had a urinary catheter for more than three days were at higher risk of developing candiduria which was consistent with the results of the current study (13).
According to the findings of this study, using two or more antibiotics is one of the main causes of the development of candiduria. A previous study reported that the most common cause of urinary tract infection with Candida is antibiotic use. Most hospitalized patients receive broad-spectrum antibiotics, which adversely affect their immune systems and promote the growth and proliferation of yeasts. This leads to the emergence of different fungal diseases, such as candiduria (14). Candida glabrata was found to be the main cause of candiduria in the present study. This finding is consistent with the results of previous studies (15, 16). The most significant challenge with regard to candidiasis is the increased rate of infections caused by non-albicans Candida species, which may be attributed to resistance to common antifungal drugs and the increasing use of immunosuppressive medications (5).
The findings of this study demonstrated that Candida species were highly susceptible to caspofungin and amphotericin B, and fluconazole and itraconazole drug resistance was commonly seen in Candida isolates. The results of antifungal susceptibility analysis indicated that all tested C. albicans, C. parapsilosis, and C. tropicalis isolates were sensitive to caspofungin, which was consistent with the findings of a study conducted by Katragkou et al. (17) They concluded that this drug can be one of the most effective antifungal drugs against various Candida species (17). Singla et al. performed a study on urine samples of 24 patients admitted to the ICU. The drug susceptibility analysis results showed that all isolates were sensitive to amphotericin B, which was pretty consistent with the results of the current study (18). Because candiduria is more common in patients hospitalized in the nephrology and ICU wards, timely and proper interventions are needed to manage contributing factors such as uncontrolled use of broad-spectrum antibiotics, prolonged hospitalization, and continued catheterization.
It is proposed that future studies with larger sample sizes and more antifungals be conducted because the results of antifungal susceptibility testing can be beneficial in the patient management and timely appropriate antifungal therapy. Candida glabrata was found to be the leading cause of candiduria. Due to the increasing antifungal resistance in this species, effective management of patients is of great concern. Therefore, it is crucial to perform drug sensitivity analysis in the mycology departments of hospitals on fungal specimens isolated from hospitalized patients with impaired and attenuated immune systems. Caspofungin showed significant antifungal efficacy against the isolates examined in this investigation. Unfortunately, however, the entire echinocandin class of drugs have a major pharmacokinetic disadvantage: They have extremely poor glomerular filtration or tubular secretion in vivo, leading to sub-therapeutic antifungal concentrations in the urine. Accordingly, caspofungin and all echinocandins are usually excluded from the antifungal treatment regimens for candiduria, albeit their efficacy in in vitro studies.