1. Background
In late December 2019, COVID-19 was first reported in Wuhan City, Hubei Province, China, causing high morbidity and mortality worldwide (1, 2). COVID-19 has a wide range of clinical severity ranging from asymptomatic infections to serious consequences such as organ failure and death (3). COVID-19 patients usually exhibit various symptoms, including fever, cough, muscle weakness, fatigue, headache, chest pain, respiratory distress, pneumonia, or death (4). However, some people infected with SARS-CoV-2 do not progress symptoms, which is a significant source of transmission, as well as a potential challenge to avoid the spread of the disease within the population (5, 6). SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) is a reference standard technique for diagnosing COVID-19. However, studies have shown that serology tests are useful for screening IgM/IgG antibodies, improving the control of asymptomatic individuals, and being, beyond any doubt, convenient public health interventions (7, 8).
Among Middle Eastern countries, Iran was one of the first countries to report SARS-CoV-2, and we saw a rapid increase in cases over the nation. In any case, early outbreaks within the nonappearance of a seroprevalence appear that the actual rate of disease in Iran remained obscure (8, 9). From the second half of November 2020, more than 800000 affirmed cases and 42000 COVID-19-related passing were reported. Early in the outbreak, 2 COVID-19-related deaths were reported in Qom (8, 10). Because of the high case numbers and expanded numbers of COVID-19 patients in healing centers, prohibiting all mass gatherings (restaurant closures, etc.) began in February 2020. Be that as it may, the facilitating of beginning lockdown limitations in April possibly lead to the moment SARS-CoV-2 wave in different cities in June 2020. Hence, the study of seroprevalence among the population is strongly required to supply a sign of the extent of the population that is not nonetheless infected and needs to arrange future medical care.
2. Objectives
In the current study, the prevalence of SARS-CoV-2 antibodies among the low-risk and high-risk individuals in Shiraz, Iran, was investigated in the initial wave of natural disaster. In addition, we assessed the seroprevalence in individuals at high risk of close contact with COVID-19.
3. Methods
3.1. Patients
We performed a cross-sectional study (including serological tests for anti-SARS-CoV-2 antibodies) to evaluate the seroprevalence of SARS-CoV-2 infection between the 2 groups according to the risk of exposure to COVID-19 patients (including 162 low-risk subjects who were normal populations and 204 of COVID-19 exposure staff employed in Namazi hospitals in Shiraz, Iran, between April and August 2020 (2 months after reporting the first COVID-19 infection cases in the country). The study was performed at the Corona Laboratory of Microbiology Research Center (CMRC), Shiraz University of Medical Sciences, Shiraz, southern Iran. The Data related to age, sex, risk of exposure to COVID-19 patients were collected in low-risk and high-risk groups. The presence and types of signs and symptoms of COVID-19 including cough, fever, sore throat, shortness of breath, sneezing, nasal congestion, runny nose, vomiting, diarrhea, anorexia, headache, vertigo, eye redness, muscle pain, fatigue, joint pain and shivering, experienced during 12 weeks were collected by a questionnaire.
3.2. Sample Collection and Testing
Samples were prepared using 2 sterile Dacron separate swabs from the throat and nose of health care workers (HCWs) and outpatient population suspected of SARS-CoV-2 infection. To transfer the clinical sample, a sterile virus transport medium containing antifungal and antibacterial supplements was used. We avoid repeated freezing and thawing of the clinical sample. The sources of the data obtained from a respective internet site of serum samples (http://coronalab.sums.ac.ir) were collected and stored at -70°C until we had the enzyme-linked immunosorbent assay (ELISA) tests available (end of August 2020). The presence of SARS-CoV-2 antibodies was assessed by IgG antibody detection using a set of COVID-19 commercial serologic quantitative tests (antigenic target nucleocapsid protein) of SARS-CoV-2 IgM (nucleocapsid protein), including ELISA, according to the instructions of the manufacturers. Quantitative detection of both IgM and IgG antibodies anti-SARS-CoV-2 was carried out using Pishtaz Teb (PT-Q SARS-COV IgG-96 and PT-Q SARS-COV IgMcap-96, Iran) SARS-CoV-2 ELISA Kits.
3.3. Statistical Analysis
The analysis was carried out using SPSS version 22 (SPSS Inc., Chicago, Ill, USA). Descriptive statistics were used to present the frequencies and percentages. The normal distribution data are presented as means and SDs. The values were analyzed using logistic regression with the corresponding 95% CI and chi-square (χ2) test. Finally, we assessed the independent data for SARS-CoV-2 infection. P values less than 0.05 were considered statistically significant.
4. Results
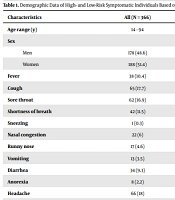
Among 704 individuals who were contacted across 2 COVID-19 diagnostic centers (CMRC and PARS laboratory center) between July to September 2020, all people first agreed to participate in the study. However, 338 participants did not give their basic demographic data (such as age) and symptoms and were excluded from the study. Among the 366 individuals included in the analysis, 204 (male 44.1% and female 55.9%) had occupations with the highest exposure to COVID-19, and 162 (male 54.3% and female 45.7%) were recruited from the general population. The mean age was 38.28 ± 9.71 and 38.63 ± 12.32 years in the high-risk and low-risk populations, respectively. Table 1 shows the putative characteristics of the participants by symptom.
| Characteristics | All (N = 366) | High Risk (N = 204) | Low Risk (N = 162) |
|---|---|---|---|
| Age range (y) | 14 - 94 | 23 - 77 | 14 - 94 |
| Sex | |||
| Men | 178 (48.6) | 90 (44.1) | 88 (54.3) |
| Women | 188 (51.4) | 114 (55.9) | 74 (45.7) |
| Fever | 38 (10.4) | 3 (1.5) | 35 (21.6) |
| Cough | 65 (17.7) | 24 (11.8) | 41 (25.3) |
| Sore throat | 62 (16.9) | 18 (8.8) | 44 (27.2) |
| Shortness of breath | 42 (11.5) | 9 (4.4) | 33 (20.4) |
| Sneezing | 1 (0.3) | 0 | 1 (0.6) |
| Nasal congestion | 22 (6) | 2 (1) | 20 (12.3) |
| Runny nose | 17 (4.6) | 14 (6.7) | 3 (1.8) |
| Vomiting | 13 (3.5) | 7 (3.4) | 6 (3.7) |
| Diarrhea | 34 (9.3) | 9 (4.4) | 25 (15.4) |
| Anorexia | 8 (2.2) | 0 | 8 (4.9) |
| Headache | 66 (18) | 30 (14.7) | 36 (22.2) |
| Vertigo | 13 (3.5) | 2 (1) | 11 (6.8) |
| Eye redness | 2 (0.5) | 1 (0.5) | 1 (0.6) |
| Muscle pain | 75 (20.5) | 32 (15.7) | 43 (26.5) |
| Fatigue | 32 (8.7) | 23 (11.3) | 9 (5.5) |
| Joint pain | 9 (2.4) | 0 | 9 (5.5) |
| Shivering | 32 (8.7) | 3 (1.5) | 29 (17.9) |
a Values are expressed as No. (%).
The seroprevalence of anti-SARS-CoV-2 antibodies was 33.33% (122/366). The immunoglobulin test for SARS-CoV-2 revealed that 50 (13.66%) and 72 (19.67%) subjects had IgG and IgM antibodies against the virus, respectively. A total of 26/204 (11.2%) and 28/204 (13.6%) of the high-risk group had IgM and IgG, respectively. The low-risk group had IgM and IgG of about 24 (14.8%) and 44 (27.2%), respectively (Table 2). There was no significant difference between men and women in both groups in positivity. In 122 seropositive cases, muscle pain 78 (11%), cough 70 (9.9%), headache 68 (9.6%), sore throat 65 (9.2%), fever 40 (5.7%), and nasal congestion 22 (3.1%) were the most commonly reported symptoms (Table 1).
| Groups | Total | Female | Male | P Value |
|---|---|---|---|---|
| High risk (N = 204) | ||||
| Positive (IgM) | 26 (12.7) | 13 (50) | 13 (50) | 1.0000 |
| Negative (IgM) | 178 (87.3) | 101 (56.7) | 77 (43.3) | 0.077 |
| Positive (IgG) | 28 (13.7) | 14 (50) | 14 (50) | 1.0000 |
| Negative (IgG) | 176 (86.3) | 100 (56.8) | 76 (43.2) | 0.074 |
| Low risk (N = 162) | ||||
| Positive (IgM) | 24 (14.8) | 12 (50) | 12 (50) | 1.0000 |
| Negative (IgM) | 138 (85.2) | 62 (44.9) | 76 (55.1) | 0.234 |
| Positive (IgG) | 44 (27.2) | 21 (47.7) | 23 (52.3) | 0.763 |
| Negative (IgG) | 118 (72.8) | 53 (44.9) | 65 (55.1) | 0.272 |
a Values are expressed as No. (%).
The high-risk group had the highest frequency of positive IgG tests associated with age groups over 30 years. The frequency of the positive IgM/IgG test related to the age groups was not significantly different in the high-risk and low-risk groups (Table 3). The multivariate logistic regression result in Table 4 showed that the most significant association was observed between headache [OR = 0.312 (95% CI, 0.136 - 0.717)], cough, and [OR = 0.427 (95% CI, 0.182 - 1.004)] and a positive anti-SARS-CoV-2 antibody test.
| Age Groups | High Risk | Low Risk | P Value |
|---|---|---|---|
| IgM positive | |||
| < 30 | 7 (58.3) | 5 (41.7) | 0.875 |
| 30 - 40 | 6 (37.5) | 10 (62.5) | 0.186 |
| 40 - 50 | 11 (68.8) | 5 (31.2) | 0.646 |
| 50 - 60 | 2 (50) | 2 (50) | 0.626 |
| > 60 | 0 | 2 (100) | 0.429 |
| IgG positive | |||
| < 30 | 3 (27.3) | 8 (72.7) | 0.037 |
| 30 - 40 | 12 (42.9) | 16 (57.1) | 0.277 |
| 40 - 50 | 11 (52.4) | 10 (47.6) | 0.217 |
| 50 - 60 | 2 (28.6) | 5 (71.4) | 0.142 |
| > 60 | 0 | 5 (100) | 0.084 |
a Values are expressed as No. (%).
| Symptoms | IgM | IgG | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable Analysis; OR (95% CI) | P Value | Multivariable Analysis Adjusted; OR (95% CI) | P Value | Univariable Analysis; OR (95% CI) | P Value | Multivariable Analysis Adjusted OR (95% CI) | P Value | |
| Fever | 0.525 (0.219 - 1.257) | 0.148 | 0.422 (0.196 - 0.909) | 0.027 | ||||
| Cough | 0.418 (0.197 - 0.883) | 0.022 | 0.427 (0.182 - 1.004) | 0.051 | 0.305 (0.157 - 0.592) | 0.000 | 0.422 (0.182 - 0.979) | 0.045 |
| Sore throat | 0.908 (0.402 - 2.050) | 0.816 | ||||||
| Shortness of breath | 0.625 (0.264 - 1.475) | 0.283 | 0.439 (0.209 - 0.924) | 0.030 | ||||
| Nasal congestion | 0.345 (0.128 - 0.924) | 0.036 | 0.098 (0.037 - 0.261) | 0.000 | 0.160 (0.055 - 0.460) | 0.001 | ||
| Runny nose | 0.864 (0.234 - 3.191) | 0.827 | ||||||
| Vomiting | 0.604 (0.157 - 2.329) | 0.464 | ||||||
| Diarrhea | 0.843 (0.318 - 2.235) | 0.731 | ||||||
| Anorexia | 0.294 (0.066 - 1.298) | 0.106 | 0.163 (0.037 - 0.713) | 0.016 | ||||
| Headache | 0.433 (0.207 - 0.908) | 0.027 | 0.312 (0.136 - 0.717) | 0.006 | 0.469 (0.242 - 0.907) | 0.024 | 0.414 (0.178 - 0.967) | 0.042 |
| Vertigo | 0.606 (0.157 - 2.342) | 0.318 (0.101 - 1.001) | 0.050 | |||||
| Muscle pain | 0.924 (0.430 - 1.987) | 0.840 | 0.435 (0.228 - 0.833) | 0.012 | ||||
| Fatigue | 0.663 (0.250 - 1.603) | 0.335 | ||||||
| Joint pain | 0.646 (0.128 - 3.269) | |||||||
| Shivering | 0.617 (0.240 - 1.584) | 0.315 | 0.256 (0.115 - 0.569) | 0.001 | ||||
5. Discussion
This study conducted between July and September 2020 and describes the evaluated prevalence of SARS-CoV-2 antibodies in 204 high-risk (HCWs) occupations at Namazi Hospital and also 162 low-risk individuals who referred to the hospital’s Contagious Diseases department from the start to the peak of the COVID-19 pandemic in Shiraz. In the present study, the seroprevalence of SARS-CoV-2 IgG and IgM antibodies was 26.4% among HCWs. Also, when serological results were compared with symptom results to analyze active or past exposure to SARS-CoV-2, no significant difference was observed between the high-risk and low-risk groups. Among 204 demographically representative high-risk individuals, there was an overall adjusted SARS-CoV-2 seroprevalence of 0.26%. The important symptoms associated with infection were cough, headache, and muscle pain in individuals with confirmed COVID-19 infection. In addition, seroprevalence was not significant in older and adult populations in the high-risk group. In the study by Mortezagholi et al., in 16.7% (5/30) whose real-time PCR test was undetectable, their serology test was IgM/IgG positive, indicating the importance of serological and molecular tests together with more efficient approaches to finding the case, as in other studies were mentioned (11-14).
These results reveal that the seropositivity prevalence of anti-SARS-CoV-2 antibodies was higher in the high-risk group than in the normal population of southwest Iran, which was reported to be 5.2% by Emami et al. (15). Also, in the study by Shakiba et al., the prevalence of anti-SARS-CoV-2 antibodies was 22% in HCWs of Guilan Province (16). In a study in March 2020, 6% of HCWs in 2 Dutch hospitals were positive for SARS-CoV-2 infection. Shortness of breath, fever, and coughing were observed in 92% of HCWs (17). In another study, 249 HCWs in Nashville, Tennessee, were examined for 1 month, of which 19 (8%) serum prevalence of antibodies were reported. The Lombardi et al., was performed in an early phase (12 February to 31 March) and revealed that 139 (8.8%) of 1573 HCWs had a positive non-structural proteins (NSPs) (18); the Sandri et al., was conducted between 28 April and 16 May and detected a similar prevalence (8%) in 2872 HCWs of 3 hospitals for SARS-CoV-2 antibodies (19, 20). These studies are in line with our study.
In the present study, IgM and IgG-specific SARS-CoV-2 antibodies were found to be present in 24 and 44% of the general population as a low-risk group, respectively. As the level of precaution for HCW was higher than that of the general population, the lower SARS-CoV-2 seroprevalence seems rational. We find that the prevalence was similar among males and females in the general population. Other study investigated among the general population and estimated that the prevalence of anti-SARS-CoV-2 antibodies obtained 2.7% in Milan, Italy (21), 5% in Spain (22) 7.6% in Daegu, South Korea (23), 4% in California (21) and 1.7% in Luxembourg (24). In similar studies in Pakistan (25) and Spain (26), the seroprevalence was 8.3 and 9.3%, respectively. Overall, 366 subjects (74.59%) reported suffering from at least one of the COVID 19 symptoms. The most recent symptoms significantly related to COVID-19 in the 2 groups include cough, fever, headache, and muscle pain.
In general, the differences observed in the prevalence of SARS-CoV-2 in various studies may be due to the characteristics of the tests (sensitivity and specificity, type of test, and the viral antigen applied in these tests), and COVID-19 infection rate during the sampling period. Furthermore, our results showed that the SARSCoV2-specific IgM and IgG titers of HCWs were not significantly higher than those of the general population, suggesting that neither group was protected during the initial wave of infection. There are some limitations and sources of bias that should be mentioned. The small sample size of health care professionals was small in this study; thus, further multicenter collaborative studies with larger samples are needed to confirm our findings. In addition, self-reported clinical symptoms by HCWs can reduce the accuracy of our study. Regarding the general population, we consider potential biases in a source of data that can take place because symptomatic and asymptomatic participants were not excluded by examination. This bias can increase the seroprevalence in this population.
5.1. Conclusions
RT-PCR technique remains important to identify acute infections, serological assays could be very essential for identifying the asymptomatic SARS-CoV-2 infected persons, as well as fast screening of HCWs who are at risk of virus infection via their direct and close contact exposure. Serological tests are possible for rapid screening of medical personnel, which is critical for public health policies for management of the COVID-19In addition, the combination of serology and molecular techniques may improve the efficiency of the case-finding approach in COVID-19 epidemiological studies, which is important for public health policies for managing COVID-19. Serological assay in populations can provide better insights into COVID19 epidemiology and assist policymakers in devising an effective strategy for combating the SARS-CoV-2 epidemic in the country.