1. Background
Pseudomonas aeruginosa is an opportunistic Gram-negative pathogen, considered the most common cause of nosocomial infection and one of the top causes of ventilator-associated pneumonia (VAP) (1). The source of P. aeruginosa pneumonia is mainly nosocomial, and it is a hospital-acquired infection. However, it can also be a community-acquired infection (CAI), primarily occurring in patients with underlying comorbidities (2, 3). Pseudomonas aeruginosa can cause infection in almost any body part; the one with the worst prognosis is blood, resulting in bloodstream infection (BSI) (4). BSI occurs mainly in immunodeficient patients and results in an annual mortality rate of 40% (5, 6). The pulmonary focus of infection, substance abuse, and comorbidities, such as cancer, cardiac, liver, and lung diseases, are considered the main risk factors for P. aeruginosa BSI and worse patients’ prognosis (7).
One of the main concerns about this pathogen is multidrug resistance (MDR), as it is the top cause of antibiotic prescription at intensive care units (ICUs) and requires adequate antibiotic therapy to reduce the mortality rate of these patients (8-10). Most combinations of antibiotics fail to treat P. aeruginosa BSI, and 70% are considered extensively drug-resistant (XDR) (11, 12). As inappropriate therapy is the central cause of mortality in patients with P. aeruginosa infection, it is suggested to perform a combination antibiogram test to identify the most possibly appropriate antibiotic, especially in patients with P. aeruginosa BSI (13, 14).
2. Objectives
In the present study, we aimed to evaluate the frequency of P. aeruginosa during a three-year period in a referral hospital in Northeast Iran and investigate its risk factors, clinical symptoms, and laboratory signs, as well as patients’ outcomes.
3. Methods
3.1. Study Design
This retrospective cross-sectional study included all cases of BSI with complete records admitted to different wards of the Imam Reza Hospital, affiliated with the Mashhad University of Medical Sciences, Mashhad, Iran, during 2012 - 2015. The researcher reviewed the laboratory records of patients, evaluated the results for finding eligible patients, and recorded their information in the study checklist. The patients' codes were recorded, and their names were not disclosed to maintain the confidentiality of their information. The recorded information included age, sex, clinical symptoms, risk factors, underlying diseases, antibiotic resistance, and infection sites. The evaluated risk factors included history of hospital admission, intubation, and surgery before blood culture, history of using injectable drugs at home (like insulin), and history of renal dialysis, corticosteroid and antibiotic therapy, and vascular catheter. The underlying diseases included hypertension (HTN), diabetes mellitus (DM), cardiovascular accident (CVA), ischemic heart disease (IHD), congestive heart failure (CHF), end-stage renal disease (ESRD), hyperlipidemia (HLP), tuberculosis, polymyositis, multiple myeloma (MM), cancer, hepatitis B, and human immunodeficiency virus (HIV).
The physician diagnosed BSI according to the results of clinical and laboratory examinations. The clinical symptoms included fever (> 37.7°C), shivering, cough, tachypnea, and impaired consciousness. The recorded laboratory parameters included leukocytosis (> 12000/mm3; normal 4,500 - 11,000), leukopenia (< 4000/mm3; normal: 4,500 - 11,000), neutropenia (< 500; normal: 1,500 - 8,000/mm3), positive C-reactive protein (CRP) (> 10 mg/L; normal ≤ 10 mg/L), and pyuria (< 10; normal 1 - 4 cells/h.p.f) with or without bacteriuria (> 105 CFU/mL in non-catheterized specimen; > 103 cfu/mL in catheterized specimen) in the urine examination. The estimated sedimentation ratio (ESR) with values ≤ 30 mm/h was considered normal, 30 - 70 mm/h moderately high, and ≥ 70 mm/h increased ESR. Appropriate treatment was defined as administering at least one of the antibiotics found sensitive in the antibiogram test. The patient admission ward and sensitivity or resistance of P. aeruginosa to antibiotics were recorded according to the results of the antibiogram test. The patients' outcome was recorded as dead or alive during hospitalization. The patients' P. aeruginosa BSI was considered nosocomial if hospitalized (at least two days before blood culture) and CAI otherwise.
3.2. Statistical Analysis
The descriptive results were presented as frequency (percentage) and mean ± standard deviation (SD) for categorical and numeric variables, respectively. The chi-square test was used to compare categorical variables between the subgroups. The statistical software IBM SPSS Statistics for Windows, version 21.0 (IBM Corp. 2012. Armonk, NY: IBM Corp) was used for the statistical analysis. P values < 0.05 were considered statistically significant.
4. Results
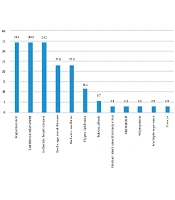
A total of 35 patients were included in the study, of whom 16 (45.7%) were women and 19 (54.3%) were men. Fever was the most common symptom (85.7%), followed by tachypnea (40%), shivering (22.9%), and reduced level of consciousness (22.9%). Among all the patients, 16 remained alive, and 19 died. The laboratory tests results showed that of the patients, 20 (57.1%) had leukocytosis, three (8.6%) had leukopenia, 14 (40%) had positive CRP; among patients with ESR test results, two (12.5%) had low ESR, six (37.5%) had moderate ESR, eight (50%) had high ESR levels, five (17.9%) had pyuria. Urine test showed that seven (25%) had pyuria + bacteriuria, and eight (22.9%) had positive urine culture. The frequency of the hospital wards and the admitted patients is shown in Figure 1. As shown, the burn unit was the most frequent ward in the hospital.
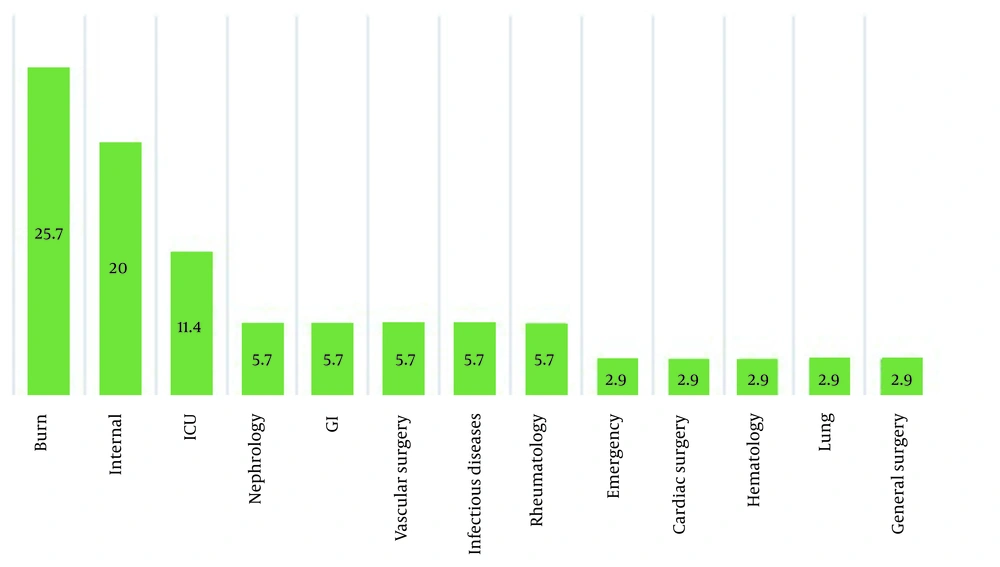
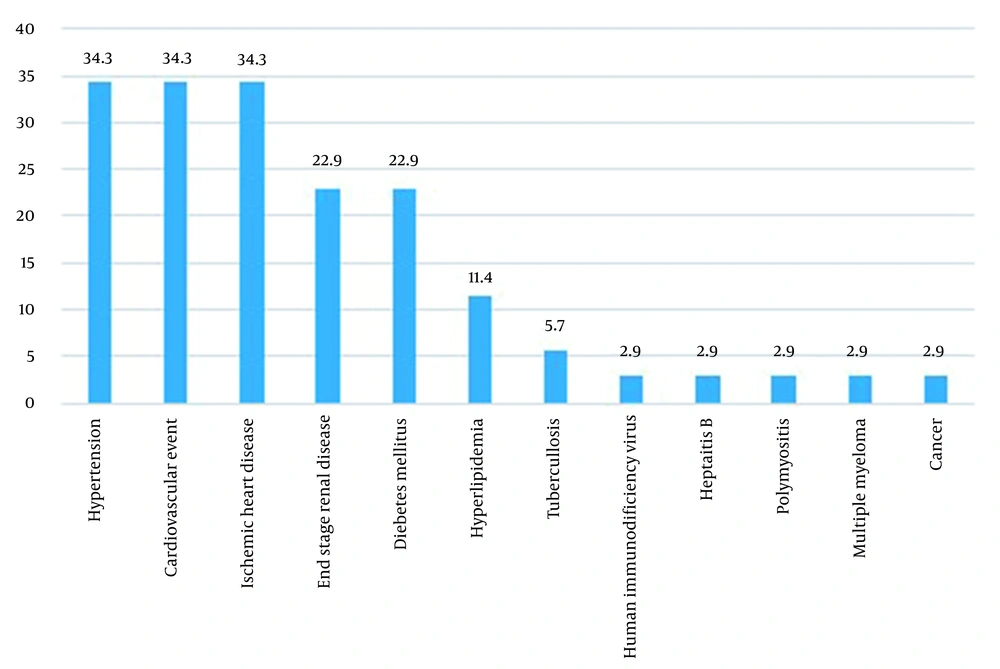
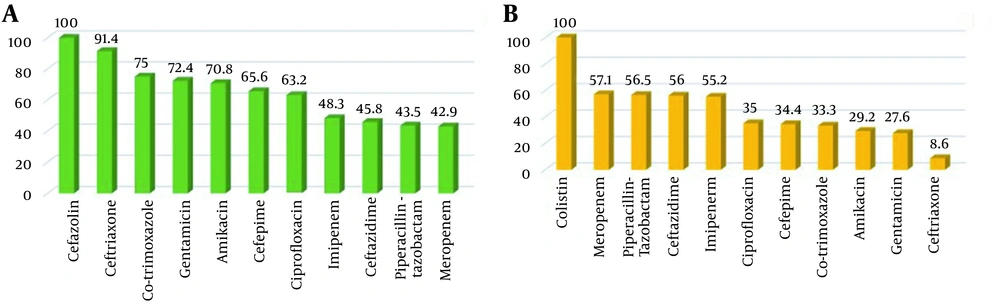
The frequency of the patients' underlying diseases is shown in Figure 2. As shown, HTN, CVA, and IHD had the highest frequency (34.3% each), followed by ESRD and DM (22.9% each). As shown in Figure 3 P. aeruginosa had a high resistance to multiple antibiotics (first-generation cephalosporin, non-anti pseudomonas cephalosporin, co-trimoxazole aminoglycoside, 4th generation cephalosporin, quinolone, anti-pseudomonas carbapenem (imipenem), anti-pseudomonas cephalosporin, piperacillin-tazobactam, and carbapenem (meropenem) in order of decreasing resistance) and was sensitive only to few antibiotics.
As indicated in Table 1, only the deceased group required intubation, while none of the patients in the alive group required intubation (P = 0.014). The frequency of appropriate treatment was significantly higher in the alive group than in the deceased group (66.7% vs. 27.8%; P = 0.02). However, the rest of the variables were not different between the dead and alive patients. Among the clinical symptoms, only the frequency of tachypnea was different between the groups and significantly higher in the deceased patients (P = 0.003; Table 1). Twenty-four patients (68.6%) had a nosocomial infection. As shown in Table 2, there was a significant difference in the frequency of death, and most patients with nosocomial infection passed away (66.7%), compared with 27.3% of patients with CAI (P = 0.03). All nine patients with a history of burning had a nosocomial infection, and none of the patients with CAI had a history of burning (P = 0.01). The frequency of shivering and decreased consciousness was also different between the groups (both P = 0.03; Table 2).
| Variables and Categories | Total (N = 35) | Alive (N = 19) | Deceased (N = 16) | P Value c |
|---|---|---|---|---|
| Positive history of admission | 31 (89) | 14 (87.5) | 17 (89.5) | 0.85 |
| Positive history of intubation | 6 (17.1) | 0 | 6 (31.6) | 0.014 |
| Positive history of using injectable drugs at home (insulin) | 6 (17.1) | 2 (12.5) | 4 (21.1) | 0.50 |
| Positive history of hemodialysis | 12 (34.3) | 5 (31.3) | 7 (36.8) | 0.72 |
| Positive history of corticosteroid therapy | 2 (5.7) | 1 (6.3) | 1 (5.3) | 0.90 |
| Positive history of antibiotic use in the past three months | 10 (28.6) | 5 (31.3) | 5 (26.3) | 0.74 |
| Positive history of surgery | 18 (51.4) | 6 (37.5) | 12 (63.2) | 0.13 |
| Positive history of burning | 9 (25.7) | 3 (18.8) | 6 (31.6) | 0.38 |
| Positive history of vascular catheter before blood culture | 15 (42.9) | 6 (37.5) | 9 (47.4) | 0.55 |
| Number of antibiotics received for treatment | 0.96 | |||
| One | 6 (17.6) | 3 (20) | 3 (16.7) | |
| Two | 20 (58.8) | 9 (60) | 11 (61.1) | |
| Three | 7 (20.5) | 3 (20) | 4 (22.2) | |
| Received appropriate treatment | 10 (66.7) | 5 (27.8) | 0.02 | |
| Clinical symptoms | ||||
| Fever | 30 (85.7) | 14 (93.8) | 15 (78.9) | 0.21 |
| Tachypnea | 14 (40) | 2 (13.2) | 12 (63.2) | 0.003 |
| Decreased consciousness | 8 (22.9) | 3 (20) | 5 (26.3) | 0.66 |
| Shivering | 8 (22.9) | 3 (15.8) | 5 (31.25) | 0.94 |
| Cough | 5 (14.3) | 1 (6.7) | 4 (21.1) | 0.24 |
a Values are expressed as No. (%).
b Considered significant at P values < 0.05.
c The results of the chi-square test.
| Variables and Categories | Community-Acquired Infection (N = 11) | Nosocomial Infection (N = 24) | P Value c |
|---|---|---|---|
| Patients’ outcome | 0.03 | ||
| Dead | 3 (27.3) | 16 (66.7) | |
| Alive | 8 (72.7) | 8 (33.3) | |
| Positive history of intubation | 0 | 6 (25) | 0.068 |
| Positive history of using injectable drugs at home (insulin) | 3 (27.3) | 3 (12.5) | 0.28 |
| Positive history of hemodialysis | 6 (54.5) | 6 (25) | 0.08 |
| Positive history of corticosteroid therapy | 0 | 2 (8.3) | 0.32 |
| Positive history of antibiotic use in the past three months | 5 (45.5) | 5 (20.8) | 0.13 |
| Positive history of surgery | 4 (36.4) | 4 (58.3) | 0.22 |
| Positive history of burning | 0 | 9 (37.5) | 0.01 |
| Positive history of vascular catheter before blood culture | 5 (45.5) | 10 (41.7) | 0.83 |
| Clinical symptoms | |||
| Fever | 11 (100) | 19 (79.2) | 0.10 |
| Tachypnea | 4 (36.4) | 10 (41.7) | 0.76 |
| Decreased consciousness | 5 (45.5) | 3 (12.5) | 0.03 |
| Shivering | 5 (45.5) | 3 (12.5) | 0.03 |
| Cough | 2 (18.2) | 3 (12.5) | 0.65 |
| C-reactive protein | 0.07 | ||
| Positive | 7 (100) | 7 (63.6) | |
| Negative | 0 | 4 (36.4) | |
| Estimated sedimentation ratio | 0.41 | ||
| High | 4 (57.1) | 4 (44.4) | |
| Moderate | 3 (42.9) | 3 (33.3) | |
| Low | 0 (0) | 2 (22.2) |
a Values are expressed as No. (%).
b Considered significant at P values < 0.05.
c The results of the chi-square test.
5. Discussion
The present study provided a broad spectrum of information about the characteristics of P. aeruginosa BSI and compared the critical items between dead and alive patients, as well as between those with nosocomial infection and CAI, which have been pointed out as two of the most important outcomes in patients with P. aeruginosa BSI (15-17). As indicated by the present study’s results, the all-cause mortality rate of the studied patients was 45.71%, similar to that reported by McCarthy and Paterson in one year (5). Others have also confirmed the high mortality rate in patients with P. aeruginosa BSI (18, 19). It has been confirmed that inappropriate initial treatment is associated with higher odds of in-hospital mortality and is more frequently observed in the deceased group (20, 21).
This finding aligns with the present study's results, emphasizing the significance of appropriate initial antibiotic treatment in such patients. All the studied cases were resistant to co-trimoxazole, gentamicin, amikacin, cefepime, and ciprofloxacin; more than half of them were sensitive to imipenem, meropenem, ceftazidime, and piperacillin-tazobactam. At the same time, all the 34 isolates were sensitive to colistin. These results are in line with the previous evidence and suggest that P. aeruginosa has become resistant to antibiotics (both MDR and XDR), especially to beta-lactams (7, 22).
The significant effect of appropriate initial empirical antibiotics in bacteremia with P. aeruginosa on patient mortality has been previously emphasized, especially in immunocompromised patients, like those with cancer or neutropenia (6, 23, 24). Some have suggested beta-lactams and amikacin, ciprofloxacin, or colistin as appropriate therapies, while the most appropriate treatment should be based on the local resistance rates (25).
The present study's results showed that all patients who required intubation died. These results propose the higher disease severity and higher MDR/XDR rates in intubated cases with P. aeruginosa BSI. As the most common pathogen causing VAP, P. aeruginosa can cause severe resistant infection and result in a high mortality rate in patients admitted to ICU, especially intubated patients (26, 27). Some have suggested that more resistant serotypes of P. aeruginosa inoculate in the endotracheal tubes, which require specific antibiotics, are responsible for these patients' higher mortality rate (28). Although these results confirm the significance of P. aeruginosa VAP, our results showed a much higher mortality rate in the intubated patients (100%) compared to previous reports (26, 29). The difference could be because they considered VAP while we included patients with bacteremia. Notably, tracheal aspiration cultures showed co-infection with other pathogens, which could be another reason for the high mortality rate of these patients.
About 70% of cases with P. aeruginosa BSI had a nosocomial infection. These results align with the previous evidence, suggesting P. aeruginosa as an important nosocomial pathogen (30). Further analysis in our study showed a significantly higher mortality rate in patients with nosocomial infection compared to those with CAI (66.7% vs. 27.3%). These results, in addition to the higher rate of decreased consciousness in patients with nosocomial infection, showed that patients with nosocomial P. aeruginosa BSI had a more severe resistant infection. Shi et al., in their five-year study showed that patients with nosocomial P. aeruginosa BSI had a significantly higher MDR rate and odds of mortality (18), which is consistent with the present study’s results. Others have also approved nosocomial P. aeruginosa BSI as a significant predictor of mortality (26), confirming the current study's results.
The higher mortality rate of our patients with nosocomial P. aeruginosa BSI compared to previous reports (18) might be attributed to the higher MDR of the pathogen in our study, which has been considered a significant cause of mortality in patients with nosocomial infection (31). Another notable finding in the present study was that all patients with burn had a nosocomial infection. Others have also suggested P. aeruginosa as the most common wound infection pathogen in patients with burns, with a high MDR rate in these cases (32, 33). This finding is in line with the present study’s results, emphasizing considering P. aeruginosa during wound care in patients with burns.
Another finding with a significant difference was the frequency of clinical symptoms, including a higher frequency of tachypnea in the deceased group and a higher rate of shivering in patients with CAI. A wide range of symptoms have been proposed for P. aeruginosa infection, most of which are non-specific (34); therefore, greater attention should be paid to the appropriate diagnosis of this infection and initiation of proper treatment as soon as possible. The first study limitation was the small sample size and selection of the participants from one center, which reduced the generalizability of the results. The second study limitation was the retrospective collection of data from the medical records; any bias during data recording could affect the study’s results. The short study duration was the last limitation of the study.
5.1. Conclusions
Considering the high mortality rate of P. aeruginosa BSI and resistance to multiple antibiotics, especially in patients with nosocomial infection, it is necessary to pay greater attention to the prevention of this infection in hospitals by frequently washing hands, following personal hygiene principles (met by doctors, nurses, visitors, and anyone in contact with the patient), disinfecting hospital environment/equipment, and avoiding unnecessary prescription of antibiotics.