1. Background
Helicobacter pylori is a Gram-negative, microaerophilic spiral-shaped bacterium that infects the epithelial layer of the stomach of affected humans, causing inflammation and ulceration (1). This bacterium remains one of the most common chronic bacterial infections affecting humans, with prevalence rates varying widely among different geographical regions and ethnic groups (2, 3). Chronic gastritis following H. pylori infection is associated with an increased risk of upper gastrointestinal diseases, such as peptic ulcer disease (PUD), mucosa-associated lymphoid tissue lymphoma (MALT), and gastric malignancies (4, 5). Therefore, H. pylori eradication decreases the risk of gastrointestinal disease and is essential to promote public health, especially in highly prevalent areas of H. pylori infection worldwide (6). In other words, population-based eradication of H. pylori infection has a potential role in decreasing the incidence of gastric cancer without increasing adverse consequences (7).
Based on the American College of Gastroenterology Guideline (ACG), proton pump inhibitor (PPI)-based triple therapy is the mainstay remedy for H. pylori infection treatment and eradication (8). Specifically, PPI-based triple therapy, usually consisting of a PPI, clarithromycin, and amoxicillin, is a widely recommended regimen for H. pylori treatment in areas with low levels of clarithromycin resistance (9). Unfortunately, due to the wide use of antibiotics, the incidence of antibiotic-resistant strains is rapidly increasing, which can decrease the likelihood of H. pylori infection eradication (10). Furthermore, recent molecular studies have shown high resistance of H. pylori to antibiotics; for instance, a multicenter European study reported a 17.5% resistance to clarithromycin (11). Molecular studies conducted in Iran predicted a clarithromycin resistance of about 22.4%. Although this rate is higher than in other regions, the effect of a clarithromycin-containing regimen is similar to that in other countries (12).
Studies have indicated that the high prevalence of clarithromycin-resistant strains of H. pylori is a major challenge for successfully treating gastrointestinal infections (13). Thus, clarithromycin-resistant H. pylori infection was designated a high priority for the field of antibiotic research and development by World Health Organization (WHO) (14). Scientific evidence indicates that patient-related factors, including antibiotic resistance and poor medication compliance, are associated with eradication failure; however, there is an ambiguous relationship between eradication failure, socio-demographic status, and clinical characteristics of patients, which needs further investigation (15, 16).
2. Objectives
Our review of the H. pylori infected patients' response to the standard triple therapy showed a few studies in Iran. Therefore, we designed a population-based study to investigate the H. pylori standard triple therapy eradication rate and its relationship with patient-related factors in the Iranian population.
3. Methods
3.1. Study Design and Setting
The present retrospective study was conducted among consecutive patients referred to Imam Reza hospital's gastroenterology clinic in Mashhad, Iran, from 2017 to 2019. We included patients with H. pylori, confirmed by the endoscopic procedure and biopsy of the upper gastrointestinal tract, and those who received H. pylori triple therapy according to ACG 2017 guidelines (17). Patients with a history of H. pylori treatment, gastric surgery or gastric cancer, macrolide consumption within three months prior to this study, pregnant or lactating women, patients with renal failure, and patients who left the treatment due to an adverse drug reaction or denied doing post-treatment urea breath test (UBT) were excluded from the study. The following data were recorded for each patient: Demographic characteristics (age, gender, history of smoking, opium addiction, alcohol consumption, methamphetamine consumption, nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin use, and family history of gastrointestinal cancers), underlying diseases and related conditions (diabetes mellitus (DM) and renal failure), the results of H. pylori stool polymerase chain reaction (PCR) and UBT, and findings of gastroduodenal endoscopy and its biopsy.
3.2. Sample Size
Although the study was carried out based on the census sampling method, the minimal sample size was calculated. Due to the lack of sufficient information about the response rate of H. pylori-infected individuals to triple therapy protocol, taking into account the frequency of 50% (P = 0.50), the type I error of 5% (α = 0.05), the statistical power of 90% (β = 0.1), and the accuracy (d = 0.2 p), the minimum sample size was calculated as 260 patients using the ratio estimation formula in a community in NCSS software.
3.3. Treatment Protocol
The patients received standard H. pylori clarithromycin-triple therapy based on the ACG 2017 protocol (17). This regimen consisted of clarithromycin (500 mg) twice daily, amoxicillin (1000 mg) twice daily or metronidazole (500 mg) three times daily, and PPI (including omeprazole (40 mg) twice daily or pantoprazole (40 mg) twice daily) for 14 days. Four weeks after antibiotic therapy and 1 - 2 weeks after cessation of PPI therapy, the patients were re-evaluated for H. pylori eradication by UBT.
3.4. Statistical Analysis
Statistical analysis was conducted using SPSS version 16 software. Descriptive statistics, including frequency (percentage), mean, and standard deviation (SD) for quantitative variables, were applied to present demographic characteristics. The chi-square test and Fisher's exact test investigated the relationship between qualitative variables. An independent t-test or Mann-Whitney test was used to compare the mean quantitative variables between the two groups (positive/negative response to treatment). All tests were two-tailed at a 5% significance level.
4. Results
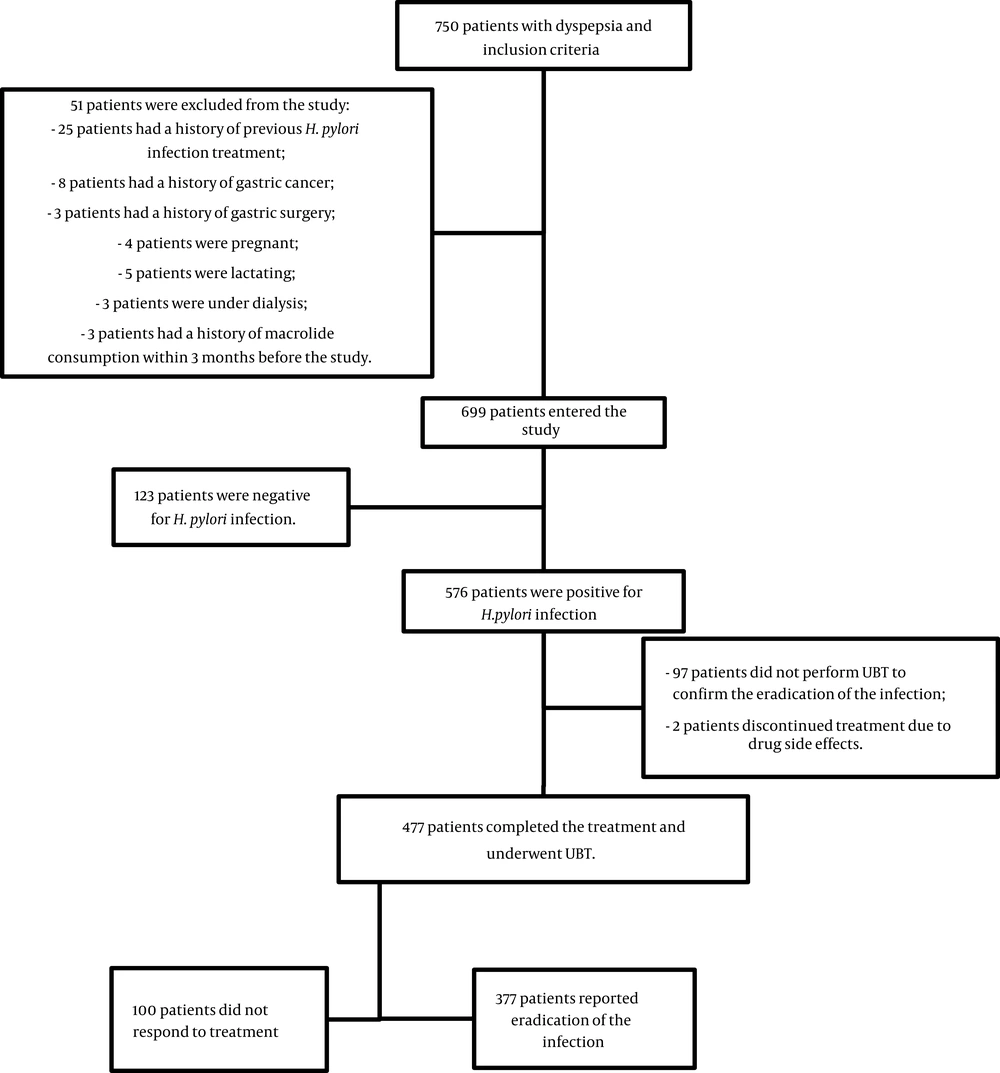
During the study period, 750 patients were referred to the gastrointestinal clinic of Imam Reza hospital with a complaint of dyspepsia. After applying the exclusion criteria, 477 patients remained in the study. Figure 1 represents the study flow diagram. Table 1 shows the demographic characteristics of the patients, including 161 males and 316 females. The patients' average ages were 48.3 ± 15.3 and 49.4 ± 15.1 years in H. pylori-positive and negative groups, respectively. Among those infected with H. pylori, 377 (79%) had a negative UBT after standard triple therapy; however, 100 (21%) patients had a positive UBT, indicating treatment failure. In detail, 126 (78.3%) males and 251 (79.4%) females responded to the treatment, which did not represent a significant difference between genders (P = 0.76). Although there were no significant differences between the treatment responses of different age groups (P = 0.483), middle-aged patients between 30 - 39 years old were more responsive.
| Characteristics and Variables | Response to Treatment | P-Value b | |
|---|---|---|---|
| Positive (N = 377) | Negative (N = 100) | ||
| Gender | 0.76 | ||
| Male | 126 (78.2) | 35 (21.8) | |
| Female | 251 (79.4) | 65 (20.6) | |
| Age (y) | 0.483 | ||
| < 30 | 47 (77) | 14 (23) | |
| 30 - 39 | 82 (83.7) | 16 (16.3) | |
| 40 - 49 | 84 (82.4) | 18 (17.6) | |
| 50 - 59 | 86 (76.8) | 26 (23.2) | |
| 60 - 70 | 46 (71.9) | 18 (28.10) | |
| > 70 | 33 (80.5) | 8 (19.5) | |
| Smoking | 0.74 | ||
| Smoking | 70 (77.7) | 20 (22.3) | |
| Non-smoking | 307 (79.3) | 80 (20.7) | |
| Alcohol | 0.91 | ||
| Alcoholic | 28 (78.3) | 8 (21.7) | |
| Non-alcoholic | 348 (79) | 92 (21) | |
| Opium | 0.89 | ||
| Addicted | 88 (78.5) | 24 (21.5) | |
| Non-addicted | 289 (79.1) | 76 (20.9) | |
| Aspirin | 0.461 | ||
| User | 60 (75.4) | 19 (24.6) | |
| Non-user | 317 (79.6) | 81 (20.4) | |
| NSAIDs | 0.663 | ||
| User | 114 (80.2) | 28 (19.8) | |
| Non-user | 263 (78.5) | 72 (21.5) | |
| Methamphetamine | 0.503 | ||
| Consumer | 17 (85) | 3 (15) | |
| Non-consumer | 360 (78.7) | 97 (21.3) | |
| Diabetes mellitus | 0.18 | ||
| Diabetic | 65 (73.8) | 23 (26.2) | |
| Non-diabetic | 312 (80.2) | 77 (19.8) | |
| Dialysis | 0.054 | ||
| Dialytic | 21 (65.6) | 11 (34.4) | |
| Non-dialytic | 365 (80) | 89 (20) | |
| Familial history of GI cancer | 0.518 | ||
| Yes | 42 (80.7) | 10 (19.3) | |
| No | 250 (77.6) | 72 (22.4) | |
Demographic Characteristics of the Study Participants in Different "Response to Treatment" Groups a
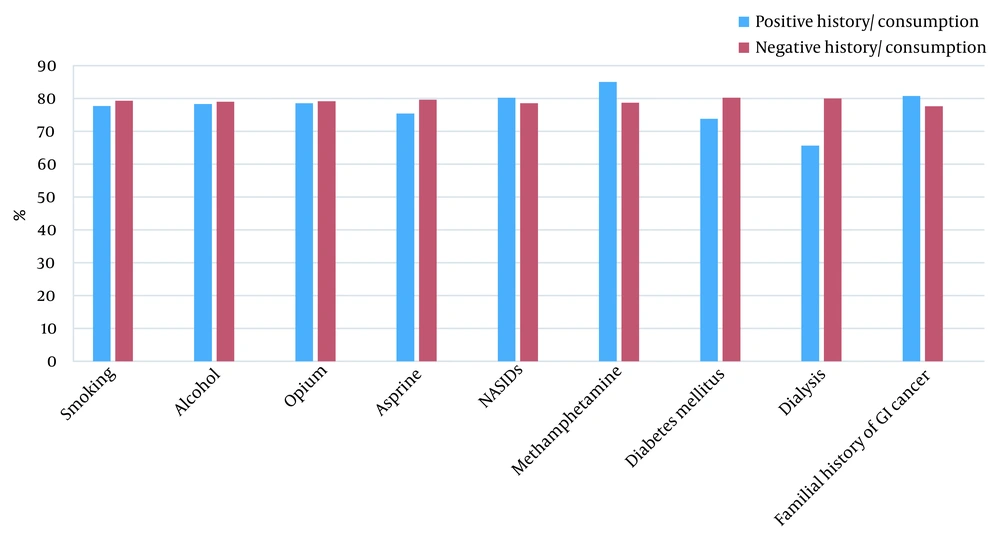
The prevalence rates of smoking, alcohol consumption, opium addiction, methamphetamine consumption, NSAIDs and aspirin use, family history of gastrointestinal cancers, diabetes, and being on dialysis are shown in Figure 2. There were no significant relationships between the response rate to treatment and smoking (P = 0.74), alcohol consumption (P = 0.91), opium addiction (P = 0.89), history of aspirin (P = 0.46) or NSAIDs (P = 0.66) use, diabetes (P = 0.18), renal failure (P = 0.054), and family history of GI malignancies (P = 0.51) (Table 1).
In investigating the relationship between the gastric endoscopic findings of the patients and their response to treatment, 69 patients had normal views, 369 patients showed non-ulcerative lesions, and 39 patients had an ulcerative lesion report. The response rates to treatment in these three groups were 73.9, 80.5, and 76.9%, respectively. There was no significant relationship between the response rate to treatment and the presence of ulcers, normal endoscopy, or other abnormal reports (P = 0.43). Moreover, the patients' gastric biopsies were evaluated for any pathological findings; 53 patients were reported as normal, and 114 had precancerous lesions, including metaplasia, dysplasia, and hyperplasia.
The response rates in the normal and abnormal gastric biopsy groups were 81.1% and 78.9%, respectively. There was no significant relationship between the presence of precancerous lesions in gastric biopsy of patients and their response to treatment (P = 0.93) (Table 2). Out of 410 patients with duodenal endoscopy reports, 275 (67.1%) reported normal endoscopic findings, 96 reported abnormal non-ulcerative endoscopic views, and 39 reported duodenal ulcers. The response rates to treatment in these three groups were 78.5%, 80.2%, and 84.6%, respectively. Furthermore, the response rate to treatment was not significantly different between patients with duodenal ulcers and other patients (P = 0.66) (Table 2).
| Variables | Response to Treatment | P-Value a | |
|---|---|---|---|
| Positive (N = 377) | Negative (N = 100) | ||
| Gastric endoscopic findings | 0.43 | ||
| Normal | 51 (73.9) | 18 (26.1) | |
| Non-ulcerative lesions | 297 (80.5) | 72 (19.5) | |
| Gastric Ulcer | 30 (76.9) | 9 (23.1) | |
| Duodenum endoscopic findings | 0.67 | ||
| Normal | 216 (78.5) | 59 (21.5) | |
| Non-ulcerative lesions | 77 (80.2) | 19 (19.8) | |
| Duodenal ulcer | 33 (84.6) | 6 (15.4) | |
| Gastric biopsy | |||
| Normal | 43 (81.1) | 10 (18.9) | 0.62 |
| Precancerous lesion | 90 (78.9) | 24 (21.1) | 0.93 |
Comparison of Gastric and Duodenal Endoscopic Findings Respecting Different Treatment Outcomes
5. Discussion
The current study aimed to investigate the H. pylori standard triple therapy outcomes, eradication rate, and the factors related to the patient's response to this therapeutic protocol in the general population of Mashhad city as a sample of the Iranian population. The results showed that more than three-quarters of the patients who received the H. pylori standard triple therapy responded to the treatment, and the infection was eradicated in their post-treatment tests. This response rate was approximately equal in both genders; moreover, there were no statistically significant differences between responsive and non-responsive patients in terms of age, a history of smoking, alcohol consumption, opium or methamphetamine addiction, using aspirin or NSAIDs, a history of diabetes mellitus or being on dialysis, and having a familial history of GI malignancies. Furthermore, there were no significant differences between the groups in the patients' gastroduodenal endoscopic views and biopsies.
Due to the importance of H. pylori infection and its treatment, some studies have been conducted worldwide to find the best therapeutic protocol and its associated factors. Nevertheless, there was too little information on this field of study in Iran. In a study by Broutet et al., 2,751 French H. pylori-positive patients were enrolled and treated with a triple therapy regimen. They reported that the failure rate was 27.9% in people under 60 and 18.6% in people over 60, showing a significant relationship between age and treatment failure rate.
Like our study, they did not find a significant relationship between gender, smoking, alcohol consumption, and treatment failure; nevertheless, their response rate was lower than ours (18). Also, another study in the Bulgarian population did not report a significant relationship between the response rate to this therapeutic regimen, age, gender, using aspirin or NSAIDs, and diabetes mellitus (19). These similarities could be due to the similarity of the lifestyles and underlying conditions in both populations; however, further studies are needed to determine this deduction.
Contrary to these studies, several studies have indicated controversial findings of the above-mentioned relationships. Yu et al. found that smoking significantly increases the failure rate of H. pylori eradication treatment. Active smoking increases the risk of H. pylori eradication failure (20). Also, De Francesco et al. investigated the predictors of H. pylori eradication outcomes with triple therapy and sequential diets and determined that increased treatment duration, smoking, and lack of the cag A gene were associated with treatment failure. In contrast, these factors were not associated with treatment failure in the sequential regimen (21).
In a study by Nam et al., the effect of type 2 diabetes on eradicating H. pylori infection was investigated among South Korean people. The eradication rate with a seven-day triple therapy regimen was obtained at 76.5% in the non-diabetic group and 73.5% in the diabetic group, but there was no significant relationship between diabetes and infection eradication (22). In contrast, Yao et al. found significant differences between the diabetic and non-diabetic Taiwanese patients' responses to the H. Pylori triple therapy regimen, although neither group achieved > 90% eradication (23). In a study by Tsukada et al., which investigated the effect of H. pylori triple therapy on dialytic patients, no significant relationship was observed between age and treatment failure. In this study, the male-to-female ratio was 6:1 in treatment failure cases and 16:16 in responsive cases, which was insignificant. Moreover, the ratio of people on dialysis to patients who did not receive dialysis was 1:6 in treatment failure and 12:20 in treatment response, but there was no significant relationship between hemodialysis and treatment failure (24). Similarly, in our study, the history of dialysis did not have a significant relationship with the response to treatment.
Another critical topic about H. pylori treatment is the duration of receiving a triple therapy regimen. Although there is some controversial evidence about the treatment period, systematic review and meta-analysis studies indicated that the 14-day triple therapy outcomes were significantly more effective than five, seven, or 10-days administration of pump inhibitor, amoxicillin, and clarithromycin-based triple therapy (25, 26). One of the most critical factors that play a predominant role in the H. pylori treatment response is antibiotic resistance. In a systematic review study conducted in 2015 in Iran, Khademi et al. investigated H. pylori antibiotic resistance between 1997 and 2013. Accordingly, in 21 studies from different parts of Iran, H. pylori's resistance to metronidazole was 61.6%, clarithromycin 22.4%, amoxicillin 16%, tetracycline 12.2%, ciprofloxacin 21%, and levofloxacin 5.3%.
This study showed that in addition to access to appropriate treatment regimens, we need to know microbial susceptibility to different treatment regimens in different geographical areas of Iran. The prevalence of infection in different regions of Iran was reported from 30.6% to 82%. Old age, being female, living in a large family, education level, hygiene level, and water contamination were reported as risk factors for infection (27). Although we investigated the H. pylori standard triple therapy outcomes, eradication rate, and its clinically related factors, it seems that molecular pathways could significantly affect clinical outcomes.
This study has several limitations. First, the adverse effects of the medications were not appropriately assessed. Second, the low number of patients with comorbidities, such as diabetes mellitus and chronic renal failure, may have affected the results. Whereas the success rate of eradication was lower than the accepted rate, whereas the success rate of eradication was lower than the accepted rate, larger studies with different kinds of regimens are also suggested for future investigations. Lastly, the analysis may be underpowered, and the result may not be completely generalizable due to the low sample size. Still, this study analyzed gastroduodenal endoscopic views of the patients, their biopsies, UBTs, and high-risk areas for gastric cancer to present the important factors which affect H. pylori treatment outcomes. Similar studies with more patients are suggested for finding the best regimen. Besides the limitations, our findings shed light on treating H. pylori-positive patients with dyspepsia.
5.1. Conclusions
We found the H. pylori standard triple therapy eradication rate near 80% in Mashhad, Iran. Although the rate was lower than the acceptable level, it was in the same range as in other countries. It also seems admissible considering the higher rate of antibiotic consumption and resistance in Iran than in other countries. The success rate of this regimen was lower in diabetic and renal failure patients but insignificant; however, larger studies are needed to find the best regimen. The eradication rate was the same in patients with precancerous lesions, ulcer dyspepsia, and non-ulcer dyspepsia; hence, this regimen can be used in these groups.