1. Background
Despite numerous advances in medicine, infection with hepatitis viruses is still a major health burden, annually affecting millions of individuals worldwide (1, 2). It can cause a variety of clinical symptoms, leading to cirrhosis and hepatocellular carcinoma (HCC). Also, there are many reports on the coinfection of different types of hepatitis viruses in patients because these types of viruses apply the same routes of transmission, such as blood transfusion, perinatal, natal, mother-to-child transmission (MTCT), etc (2, 3). According to the World Health Organization (WHO), viral hepatitis causes 1.34 million deaths per year, which is significantly higher than mortality from other viral infections (1, 4, 5). Epidemiological studies have estimated that approximately 350 million people are infected with the hepatitis B virus (HBV) for at least 6 months (6), and 2 billion individuals worldwide are infected with the virus in their lifetime (5).
It is believed that the majority of patients acquired HBV infection during their perinatal or early childhood period, especially in highly endemic regions (7). Notably, even in areas with low endemic transmission rates, infections in the perinatal period or early childhood could account for more than a third of chronic infections (8-11). Hepatitis C virus (HCV) is thought to be a major cause of chronic hepatic disorders, which could ultimately lead to the development of HCC and impose a significant economic burden on patients (12). The morbidity and mortality rates of HCV infection are considerably high, as it has affected more than 70 million people worldwide. For instance, coinfection of HIV-positive patients with hepatitis viruses dramatically increases the incidence of severe liver diseases and mortality among HIV-infected patients (13-16).
It has been reported that chronic HBV infection alone accounts for about 50% of all cases with HCC over the world, while coinfection of HCV and HBV is markedly correlated with the development of HCC, developing acute fulminant hepatitis, liver cirrhosis, chronic hepatitis, or spontaneous clearance of one or both viruses (17-20). The global prevalence of coinfection is reported to be between 1% and 18%. It should be noted that geographical regions influence the incidence of coinfection as a higher rate of coinfection was found in endemic areas for each virus (18-20). For example, the incidence of HBV and HCV is significantly higher (above 10%) in Iranian patients (5).
A number of studies have shown that patients with acute coinfection of HCV and HBV have a shorter duration of hepatitis B surface antigen (HBsAg) and delayed appearance of this antigen in their serum samples compared with patients with acute HBV mono-infection who developed a biphasic pattern of the increased activity of alanine aminotransferase (ALT; previously SGPT) (20, 21). It has been reported that the risk of MTCT of HCV is 3 - 4 times higher in women who are coinfected with HIV than those lacking AIDS (22-24). Coinfection with HIV significantly influences the natural history of HBV infection and increases the transmission risk rate, chronic hepatitis, and viral replication (25). It is estimated that 10% of HIV-infected patients are coinfected with HBV worldwide (26). To date, there is no report on the direct impact of HBV on the progression of HIV; however, evidence shows that HCV infection increases the risk of mortality in patients with AIDS, as the presence of HIV is able to increase the risk of severe hepatic disorders. Based on the epidemiological data, nearly 30% of HIV-infected patients are also afflicted with HCV (27).
Hepatitis delta virus (HDV) is a single-stranded negative-sense RNA virus that exclusively replicates in patients who are coinfected with HBV. In fact, HDV employs the HBsAg of HBV to replicate within the cells (28, 29). Infection by HDV usually results in acute or chronic hepatic disorders, and it is estimated that nearly 5% of individuals are afflicted with HDV worldwide. Clinical evidence indicates that the concurrent infection of HDV and HBV leads to a higher degree of liver involvement than those afflicted with HBV alone (29). The onset of acute HDV infection can proceed with the onset of chronic HBV infection or occur at the same time as the acute infection of HBV (coinfection) (30, 31). Since HDV infection is usually associated with the inhibition of HBV replication, few cases of postnatal HDV transmission are believed to occur in infants (32, 33).
2. Objectives
Awareness of the adverse effects of these coinfections on the clinical outcomes of viral hepatitis necessitates further effective management of the disease. In addition, infection prevention and screening are remarkable prophylactic strategies to prevent HBV reactivation. Due to the limited information available in this field, the current research was carried out to determine the prevalence of HCV-Ab, HDV-Ab, and HIV-Ab in HBV-infected women and their newborns in northeastern Iran.
3. Methods
3.1. Study Population and Sample Collection
The present cross-sectional study was performed on 50 mothers and their infants between September 2020 and January 2021 in northeastern Iran. The participants were registered as chronic HBV-positive patients (including mothers and their infants) in the Patient Detection System of Golestan University of Medical Sciences. To isolate serum, 6 mL of whole blood was collected from all participants under standard conditions and poured into sterile EDTA-containing tubes. Samples were centrifuged at 2500 rpm for 5 minutes to separate plasma specimens. Then, HBV-related markers, including hepatitis B core antibody (HBcAb), hepatitis B e antibody (HBeAb), hepatitis B e antigen (HBeAg), hepatitis B surface antibody (HBsAb), and HBsAg, were analyzed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Pishtazteb Co, Iran). Based on the results of HBV-related markers and Table 1, they were assigned to 2 experimental groups.
| HBsAg | HBsAb | HBcAb | HBeAg | HBeAb | Number of Individuals | |
|---|---|---|---|---|---|---|
| Mothers | Positive | Negative | Positive | Positive/negative | Positive/negative | 50 |
| Infants | Positive | Negative | Positive | Positive/negative | Positive/negative | 50 |
3.2. Anti-HDV, HCV, and HIV Detection
The serum samples obtained from centrifugation were used to determine anti-HCV, anti-HDV, and anti-HIV by ELISA (DIA.PRO, Milano, Italy). Also, the activity levels of aspartate aminotransferase (AST; previously SGOT), ALT, and alkaline phosphatase (ALP) enzymes were evaluated in all participants to assess liver function using commercially available kits (Pars Azmoon, IRAN). In addition to using the positive and negative controls for the set-up process of commercial kits, the plasma samples of 4 individuals were applied; they were infected with HIV/HBV, HDV/HBV, HCV/HBV, or HBV alone as positive controls for HIV, HDV, HCV, and HBV specimens, respectively. Besides, a plasma sample of a healthy subject was employed as a negative control sample.
3.3. Statistical Analysis
The statistical analysis was performed using SPSS version 20 (SPSS Inc, Chicago, Ill, USA). The difference between the 2 experimental groups was evaluated using the Fisher exact test and chi-square test where appropriate. The obtained values were expressed as mean ± SD or frequencies, according to the nature of the data. The level of the statistical significance was set at a P-value < 0.05.
4. Results
4.1. Demographic Characteristics
The majority of the participants in group 2 were male (18.9%) and single (20.9%), with a mean age of 24.12 ± 10.281 years. Most participants were illiterate (55%) and rural residents (72%). No significant difference was found between age and antibody levels against HCV and HDV; however, a significant discrepancy was detected between education and anti-HDV antibodies (95% CI, 0.113 - 0.332), as well as between place of residence and anti-HCV antibodies (95% CI, 0.313 - 0.416) in all cases.
4.2. Serological Markers and Biochemical Tests
The majority of HBsAg+ patients (88.9%) were negative for HBeAg. In addition, the mean HBcAb titers (> 1.1) in all mothers and their infants were reported to be 9.9 ± 3.37 and 10.49 ± 3.30, respectively. On the other hand, all individuals in the 2 groups were analyzed in terms of the liver function test by measuring AST, ALT, and ALP enzymes. The biochemical analysis results revealed that the average level of ALP was higher than the standard range (80 - 360 U/I), while the mean level of ALT/AST was normal in mothers (> 40 U/I).
4.3. HDV/HBV Infection
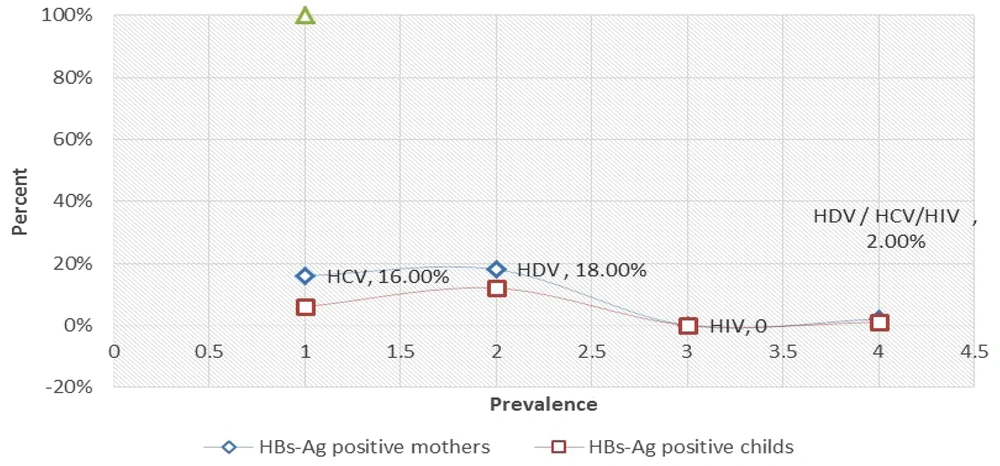
Among 100 pairs of HBsAg-positive mothers and their infants, 9 (18%) mothers and 6 (12%) infants were positive for anti-HDV antibodies. In other words, 15% of all cases were seropositive for anti-HDV. As shown in Figure 1, the rate of HDV-Ab was higher in chronic HBV-positive mothers than in their infants (95% CI, 0.527 - 4.94), while no statistically significant difference was observed between them (linear-by-linear associated test > 0.05). Also, the difference between age and the presence of HDV infection was not significant (Fisher exact test > 0.05).
4.4. HCV/HBV Infection
The prevalence of anti-HCV antibodies in participants was 11% (n = 11). The rate of anti-HCV antibodies was higher in mothers with HBV than in their infants (95% CI, 0.743 - 11.98); however, no statistically significant difference was found between them (linear-by-linear associated test > 0.05). Also, the difference between age and HCV infection was not statistically significant (Fisher exact test > 0.05).
4.5. HIV/HBV Infection
Based on the experiments performed, all participants, including mothers and their infants, were negative for anti-HIV antibodies.
4.6. HBV/HCV/HDV/HIV Infection
The prevalence of HDV/HCV in all participants was 3% during the study period. In mothers with HBV and their infants, 1 mother was coinfected with HDV and HCV, while 1 mother and her infants were coinfected with HDV and HCV (Figure 1).
5. Discussion
Epidemiological studies have demonstrated the high prevalence of viral hepatitis worldwide. Also, the high incidence of HDV/HCV hepatitis among HBV-infected individuals suggests the same transmission routes for hepatitis viruses (1, 4). In the current research, the incidence rates of HCV and HDV infections in women with chronic HBV and their infants were 11% and 15%, respectively. Moreover, infants who were positive for HBsAg had significantly higher ALP activity than their mothers who were positive for HBV. This study highlights simultaneous infection with HBV-HDV or HCV in women with chronic HBV and their infants, indicating that such a concomitant infection rapidly progresses to chronic liver diseases, liver failure, and mortality in patients. The incidence rates of HCV and HBV vary globally and depend on geographical areas and demographic characteristics (9). The prevalence of HCV-Ab among the general population of Iran was estimated to be 0.3% (34).
The seroprevalence rates of anti-HCV antibodies in healthy adults of Mashhad, Isfahan, Ardebil, Tehran, and Ahvaz, as well as male blood donors of Tabriz and infertile males of Tehran, all were negative for this virus (4, 31-35). Our results indicated that the prevalence of patients with HCV (11%) was different compared to the report of the Persian Guilan cohort study. Mansour-Ghanaei et al. demonstrated that among 10,520 samples, the prevalence rates of HCV and HBV were 0.11% (95% CI, 0.06 - 0.19) and 0.24% (95% CI, 0.16 - 0.35), respectively (11). In this regard, a study performed on 2475 subjects showed that the prevalence of anti-HCV was 5.66% (95% CI, 4.81 - 6.64%) (35). These findings are in line with our results concerning the prevalence of antibodies against HCV (11%) among all subjects.
Several lines of evidence show that HDV infection in individuals who are positive for HBsAg+ accelerates the progression of HBV-related hepatic disorders (36). In our study, the prevalence of anti-HDV in participants was 15%, which is consistent with Beguelin and colleagues, who analyzed the seroprevalence of HDV in 818 subjects with HBsAg positivity and indicated an incidence rate of 15.4% (36). Sellier et al. reported that 22 (3%) out of 742 HBV-infected women were coinfected with HDV, while 36 infants were negative for anti-HDV. Besides, 10 patients (28%) were neither infected nor protected, and 1 individual was infected with chronic HBV (33). In the present research, the prevalence of anti-HDV was 18% in women infected with HBV and 12% in HBsAg+ infants. This finding is relatively in agreement with the results of Sellier et al.
Dal Molin et al. (37) studied 126 mothers and their infants and monitored 105 exposed children for at least 12 months to assess the risk of mother-to-infant HCV transmission. According to their results, HCV infection was detected in 5 of 76 infants (6.6%) born to 69 HCV-positive mothers, while none of the 29 infants born to 26 HCV-infected mothers were positive for HCV infection. Of note, only 1 infant was positive for HCV 1 month after birth, while the remaining infants became positive 3 - 4 months after birth (37). In our study, the prevalence of HCV positivity among 100 participants was 11%, which was 7% in mothers and 4% in infants. Remarkably, in 1% of participants, 1 mother and her infant had anti-HCV simultaneously. Consistent with the findings of Khazaee et al., our results also demonstrated that the mean ages of HBV-positive mothers and their children were 50.78 ± 13.53 and 24.12 ± 10.28 years. Our findings are in line with 2 studies conducted in 2016 on 300 patients with HBV (38.61 ± 11.98 years) (38) and in 2018 on 100 HBsAg+ patients (47.44 ± 14.56) (34). Therefore, it is observed that most patients with chronic HBV are in the age range of 25 - 50 years. Therefore, paying attention to them is of special importance regarding the need for treatment.
In the present study, among mothers with chronic HBV and their infants, 11% had anti-HCV antibodies, 15% had anti-HDV antibodies, and 3% had concomitant anti-HCV and anti-HDV. Consistent with our findings, Khazaee and colleagues reported that among 300 chronic HBV-infected individuals, 11 individuals (3.7%) were coinfected with HCV, 10 (3.3%) with HDV, and 2 (0.6%) with both HCV and HDV (38). Yami et al. showed that the prevalence rates of HCV, HBV, and HIV were 0.2, 2.1, and 2.1%, respectively. They also reported that age and sex were significantly correlated with HBV and HIV infections, in which women were less likely to be infected. The risk of HBV and HIV infections increases significantly in individuals above 20 years old. Also, no significant association was found between the seropositivity for HBV/HCV/HIV and patients’ age (22). In contrast to the study of Yami et al., no positive cases of anti-HIV were observed in our study among mothers and their infants.
Mutagoma et al. demonstrated that the incidence of HBsAg positivity was 3.7% (95% CI, 3.4 - 4.0%) among 13,121 pregnant women. They reported that the prevalence of seropositivity of HIV among pregnant women who were positive for HBsAg was 4.1% (95% CI, 2.5 - 6.3%). In line with this, the frequency of individuals with concurrent infection of HBV and HIV was higher in women with an age range of 15 - 24 years than those with an age range of 25 - 49 years (odds ratio [OR] = 6.9; 95% CI, 1.8 - 27.0) (39). As opposed to the results of Mutagoma et al., the present study indicated that among all 100 participants, none of the mothers and their infants were positive for HIV and HBV. Although we did not find any significant association between sex, age, and the prevalence of anti-HCV or anti-HDV, a statistically significant correlation was found between education and anti-HDV (95% CI, 0.113 - 0.332), as well as place of residence and anti-HCV antibodies in all cases (95% CI, 0.313 - 0.416). It appears that various demographic characteristics of the populations in different areas play a significant role in variations in the prevalence of HCV and HBV (11). Our results are in agreement with the findings of Ahmed et al. (40), Makhlouf et al. (31), and Gish et al. (41).
5.1. Conclusions
HBV infection in northeastern Iran is relatively high. Also, there were significant associations between education and antibody levels against anti-HDV, as well as place of residence and antibody levels against anti-HCV in HBsAg-positive women and their infants. Therefore, it is recommended to consider the possibility of HDV or HCV infection in patients, especially in mothers and infants with HBV infection, to choose appropriate treatment methods. The diagnostic procedures of HIV, HCV, and HDV in patients with chronic HBV have challenged public and global health, and preventing MTCT is the most effective way to control the global HBV/HCV or HDV epidemic.