1. Background
Infertility is a worldwide issue that affects more than 15% of couples. Approximately 50% of infertility problems are related to males (1). A study in Iran showed that about 20% of couples are infertile, in which male factors (~70%) were significantly higher than female issues (~30%) (2). Many factors are involved in male infertility, such as genetic mutations, lifestyle, infectious diseases, medications, and idiopathic sperm abnormalities (3). It is well established that infection and inflammation are critical involved factors in male infertility (4). Microorganisms, such as bacteria, viruses, and parasites, play essential roles in men's infertility. Bacterial infections can cause sperm death, oligospermia, and asthenozoospermia. A study has shown that viral infections affect sperm parameters, especially sperm motility, with inflammation induction (5). It is well documented that the induction of inflammatory cytokines negatively impacts spermatogenesis and reproductive system function (4).
Many viruses, such as hepatitis C virus (HCV), hepatitis B virus (HBV), human immunodeficiency virus (HIV), herpes simplex virus, and cytomegalovirus (CMV), can cause male infertility (6). Several studies in Iran have indicated that CMV and HBV infections play a major role in male infertility (2, 7). It has been reported that CMV can be extracted from almost all body secretions, including vaginal and cervical secretions, seminal fluid, oropharyngeal secretions, and milk, as well as stool, urine, and blood (8). An in vitro study showed the attachment and intracellular accumulation of CMV on the head, tail, and connecting pieces of spermatozoa in infected semen samples (9). Moreover, it has been shown that the association of CMV with chronic inflammatory urogenital diseases results in male sterility (9). Another research demonstrated that CMV led to male infertility by inducing asthenozoospermia and oligospermia (5).
Hepatitis B virus transmits via genital secretions and sexual contact. This virus was established to disrupt sperm parameters and release inflammatory cytokines (7). Inflammatory cytokines are induced in the early phase of viral infections. Tumor necrosis factor-alpha (TNF-α) is an essential inflammatory cytokine, and studies have demonstrated a direct correlation between TNF-α increase and male infertility (10). A mitochondrion is the cell's powerhouse organelle that produces adenosine triphosphate (ATP) by oxidation-reduction reactions in its membrane. Membranes of mitochondrion are involved in ATP production. There is a potential membrane across mitochondrial membranes, which plays a pivotal role in ATP production. Therefore, any impairment in mitochondrial membrane potential (MMP) can affect cell function.
Pathogens impair cell performance by different pathological pathways. Impairment of MMP is one of the pathological pathways that target cell functions (11) and plays a critical role in sperm function. Moreover, it has been demonstrated that any changes in the MMP of sperm influence fertility (1). Inflammatory cytokine mediator, TNF-α, has been proven to decrease MMP, which results in asthenozoospermia and alterations in other parameters of sperm (12). Studies showed that CMV and HBV caused mitochondrial dysfunction (11, 13).
2. Objectives
As far as we know, the formerly mentioned studies evaluated the effect of HBV infection on TNF-α, MMP, and infertility separately. However, this study was designed to investigate whether viral infections decrease MMP and infertility by increasing the level of TNF.
3. Methods
3.1. Study Population
This prospective case-control study was performed on 73 men who were referred to the Reproductive Biology Research Department, Infertility Research and Treatment Center of Khuzestan, ACECR, Ahvaz, Iran, during January 2020-December 2020. The subjects were divided into groups healthy fertile (n = 22 volunteers) as the healthy control group, non-infected infertile group (n = 27), HBV-infected infertile (n = 14), and CMV-infected infertile (n = 10). According to the fifth edition of World Health Organization (WHO) guidelines, men with normal sperm parameters and no HBV-positive PCR test were considered control (healthy fertile males) (14). The infertile non-infected patients who were negative for HBV or CMV infections were considered the non-infected infertile group. Moreover, the infertile patients with positive HBV or CMV PCR tests were regarded as HBV-infected and CMV-infected infertile groups.
The inclusion criteria were infertile males (aged 33.78 ± 2.74) without childbearing twelve months after successful intercourse, while their wives did not have infertility problems. In addition, only the samples of infertile groups with asthenozoospermia entered the study. The exclusion criteria entailed infertility due to genetic disorders, bacterial infections, and testicular abnormalities (e.g., epididymis, cryptorchidism, varicocele, and testicular torsion). All the steps of the study, including sampling, analyses of sperm parameters, as well as molecular, cellular, and serological evaluations, were performed following the WHO protection protocols because of working with infected samples.
3.2. Semen Collection
The samples were collected by masturbation in a sterile tube. Briefly, patients were asked not to do intercourse seven days before giving semen samples. Samples were shipped to the laboratory at 37°C within 30 min. According to WHO guidelines, semen samples were analyzed for sperm parameters, including color, volume, liquefaction time, pH, density, total count, progressive motility (PR), progressive and non-progressive motility (PR + NP), morphology, and leukocytes (15).
3.3. Determination of Mitochondrial Membrane Potential in Semen Samples
One million sperms from fresh semen samples were used to determine MMP using a commercial kit (JC1 Mitochondrial Membrane Potential Assay Kit, Abnova Company, Taipei City, Taiwan). According to the manufacturer's instructions, specimens were washed three times by washing buffer, and then JC-1 dye (1/100 dilution) was added to the samples. Afterwards, samples were incubated in darkness at 37°C for 15 min, and finally, the fluorescent intensity was evaluated as described below using flow cytometry (BD FACSCalibur™, BD-Biosciences, USA). JC-1 formed J-aggregation in healthy cells, which displayed strong fluorescent intensity with excitation (535 nm) and emission (595 nm). JC-1 existed as monomers in apoptotic or unhealthy cells, which indicated strong fluorescence intensity with excitation at 485 nm and emission at 535 nm. The ratio of fluorescent intensity of J-aggregates to the fluorescent intensity of monomers was used as an indicator of cell health, and MMP was expressed as Red/Green or R2/R3.
3.4. Molecular Analysis of Semen Samples
The semen specimens of all studied participants (73 volunteers) were subjected to PCR to assess the presence of HBV or CMV infection. DNA was extracted from frozen semen samples using DNA extraction Kit DNP (SinaClon, Iran). The purity and concentration of extracted DNA were spectrophotometrically measured with a nanodrop (ThermoScientific, USA). Samples with purity above two were used for molecular study. To determine HBV infection, semi-nested PCR was performed using the specific primers listed below. The forward and reverse primers used for the first round of PCR were 5- ACC WTA TWC YTG GGA ACA A-3 and 5- GAY GAY GGG ATG GGA ATA CA-3, respectively. The second forward and reverse primers applied in the second round of PCR were 5- ACC WTA TWC YTG GGA ACA A-3 and 5-AGR AAM ACM CCG CCT GT-3, respectively (16).
Table 1 shows the temperature and timing of PCR steps. Nested PCR was performed using the following primers to determine CMV infection. The forward and reverse primers for the first round of PCR were 5- GCA ACG AGA ACC CCG AGA AAG -3 and 5- AAG CCA TAA TCT CAT CAG GG -3, respectively. The second forward and reverse primers utilized in the second round of PCR were 5- GCG GCA TAG AAT CAA GGA GCA CAT G -3 and 5- CAA GCC ATC CAC ATC TCC CGC -3, respectively (17). Table 2 indicates the temperature and timing of PCR steps. Amplicons were run on 2% agarose gel, and electrophoresis was completed. Finally, double-stranded DNA fragments in the gel were visualized with DNA Safe Stain (SinaClon, Tehran, Iran).
| Step & Phase | Temperature (°C) | Time |
|---|---|---|
| 1st round | ||
| Initial denaturation | 95 | 5 min |
| Denaturation | 95 | 30 sec |
| Annealing | 58 | 30 sec |
| Extension | 72 | 45 sec |
| Final extension | 72 | 5 min |
| 35 cycles | ||
| 2nd round | ||
| Initial denaturation | 95 | 5 min |
| Denaturation | 95 | 20 sec |
| Annealing | 58 | 50 sec |
| Extension | 72 | 45 sec |
| Final extension | 72 | 5 min |
| 30 cycles | ||
Abbreviation: HBV, hepatitis B virus.
| Step & Phase | Temperature (°C) | Time |
|---|---|---|
| 1st round | ||
| Initial denaturation | 95 | 5 min |
| Denaturation | 95 | 30 sec |
| Annealing | 50 | 30 sec |
| Extension | 72 | 60 sec |
| Final extension | 72 | 7 min |
| 35 cycles | ||
| 2nd round | ||
| Initial denaturation | 95 | 5 min |
| Denaturation | 95 | 30 sec |
| Annealing | 58 | 30 sec |
| Extension | 72 | 45 sec |
| Final extension | 72 | 7 min |
| 30 cycles | ||
Abbreviation: CMV, cytomegalovirus.
3.5. Measurement of TNF-α in Semen Samples
In order to determine the semen level of TNF-α, frozen semen specimens were melted at laboratory temperature and then centrifuged (5 min, 3000 g). Next, the concentration of TNF-α in the supernatant was measured using an enzyme-linked immunosorbent assay kit (Karmania Pars Gene Company, Iran), following the manufacturer's instructions. In order to calculate the concentration of TNF-α in semen, a standard curve was created utilizing different concentrations of standard solution (200, 100, 50, and 5 pg). The sensitivity of the kit was 2 pg/mL. The inter- and intra-assay accuracy of the kit were > 8% and > 3%, respectively.
3.6. Statistical Analysis
The SPSS version 23 was used to analyze the data. The Kolmogorov-Smirnov test was applied to test the normality of data. Data were expressed as mean ± SEM. One-way analysis of variance (ANOVA) and Tukey posthoc test were used. P < 0.05was considered significant.
4. Results
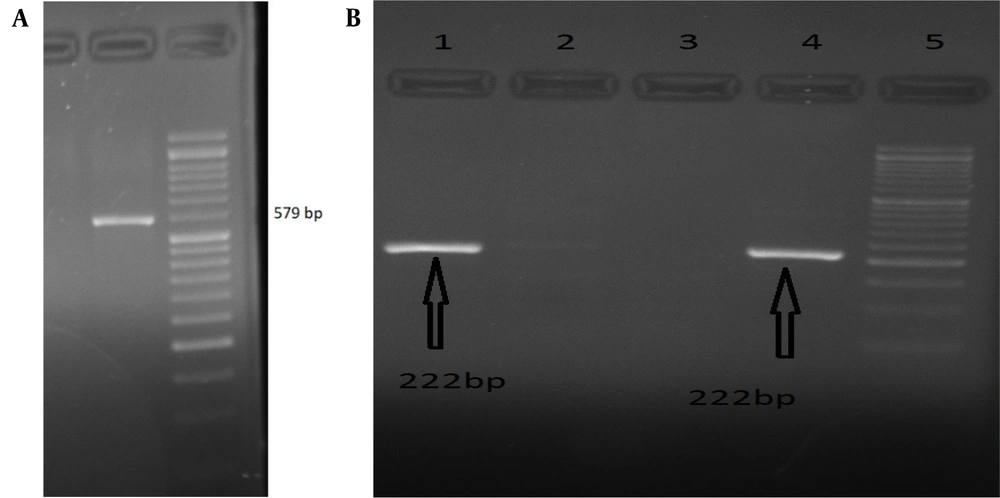
The participants of this study aged 33.78 ± 2.74 years. PCR results showed that 14 out of 73 volunteers were positive for HBV infection (Figure 1A). In addition, ten of 73 volunteers were positive for CMV infection (Figure 1B).
A, Agarose gel image of PCR for hepatitis B virus (HBV). The gel image shows that the semi-nested PCR product was 579 bp, which is an HBV amplicon. DNA 50 bp ladder; B, Agarose gel image of PCR for cytomegalovirus (CMV). The gel image shows that the nested-PCR products were 222 bp, which are CMV amplicons. DNA 50 bp ladder.
4.1. Effects of HBV and CMV Infections on Sperm Parameters
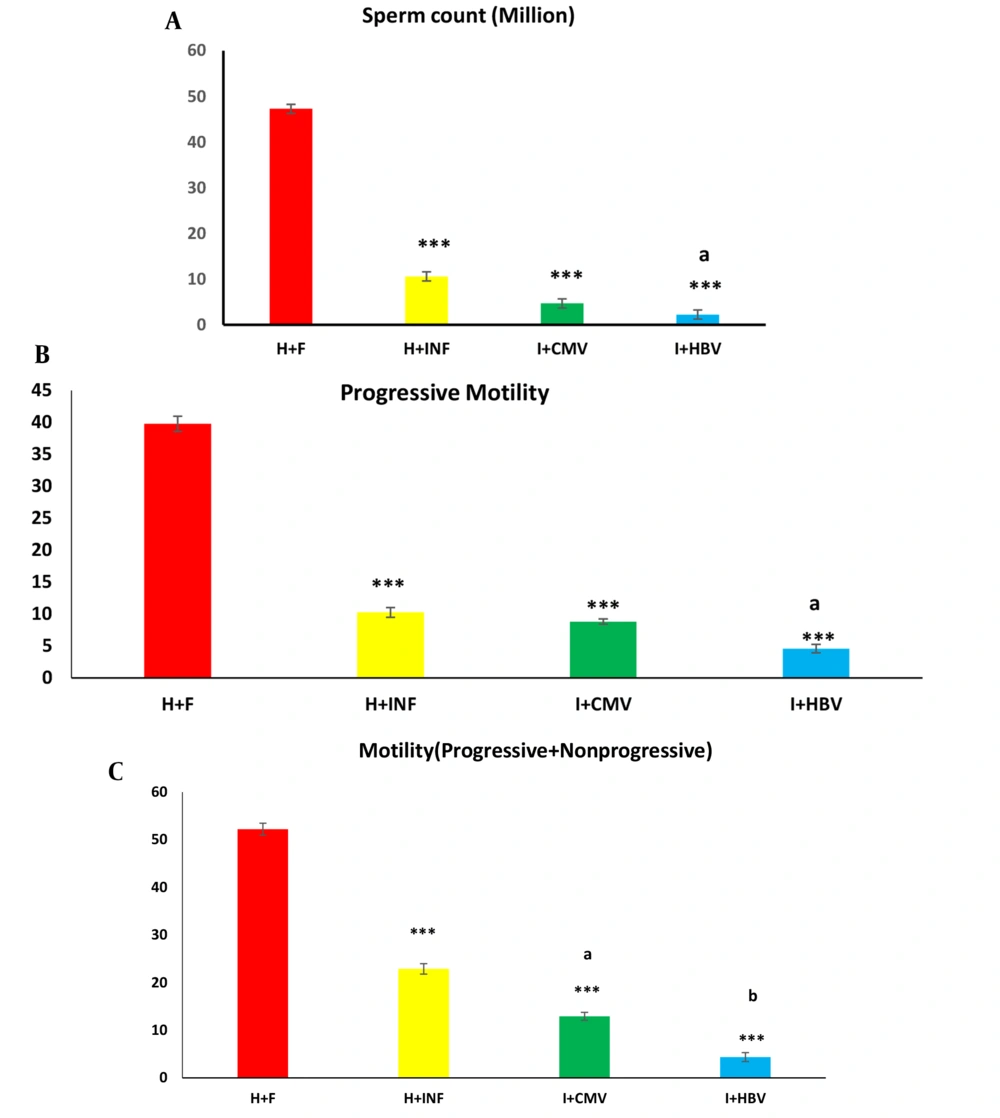
As illustrated in Figure 2A, the number of sperms in the HBV- and CMV-infected males significantly decreased compared to healthy controls (both P < 0.001). Moreover, this parameter indicated a significant decline in the HBV-infected group compared to the healthy infertile group (Figure 2A, P < 0.05). The measured PR and PR + NP significantly decreased in the infertile groups (non-infected and infected groups) compared to the healthy controls (Figure 2B and C, P < 0.001 in both cases). As demonstrated in Table 3, the highest percentage of normal sperms (normal morphology) was observed in the healthy control group. Our findings revealed that 45% of sperms showed more than 50% normality, and 55% of sperms showed more than 75% normality. Percent of sperm normality in infertile groups (non-infected and infected) was lower than 25%. In addition, the lowest normal percent of sperms was found in the HBV-infected infertile group.
Effects of hepatitis B virus (HBV) and cytomegalovirus (CMV) infections on sperm parameters. Almost all parameters, including sperm count (A), progressive motility (B), and progressive and non-progressive motility (C) are influenced by HBV and CMV infections. These parameters in the non-infected infertile group were also significantly lower than in the healthy control group. H + F, H+INF, I + CMV, and I + HBV represent the healthy control, non-infected infertile, CMV-infected infertile, and HBV-infected infertile groups, respectively. Data are expressed as mean ± SEM. One-way analysis of variance (ANOVA) and Tukey posthoc test were used to analyze the data. *** P < 0.001 versus the healthy control group; (a) P < 0.05 versus the healthy infertile group, and (b) P < 0.05 versus the CMV-infected infertile group.
| Morph (% of Normality) | H + F | H + INF | INF + CMV | INF + HBV |
|---|---|---|---|---|
| > 25 | 0 | 85 | 70 | 50 |
| < 25 | 0 | 0 | 0 | 0 |
| 50 < | 45 | 0 | 0 | 0 |
| < 75 | 55 | 0 | 0 | 0 |
Abbreviations: HBV, hepatitis B virus; CMV, cytomegalovirus; H+F, healthy control; H+INF, non-infected infertile; I+CMV, CMV-infected infertile; I+HBV, HBV-infected infertile.
aMorphology in the non-infected infertile group was also lower than in the healthy control group.
4.2. Effect of HBV and CMV Infections on Mitochondrial Membrane Potential
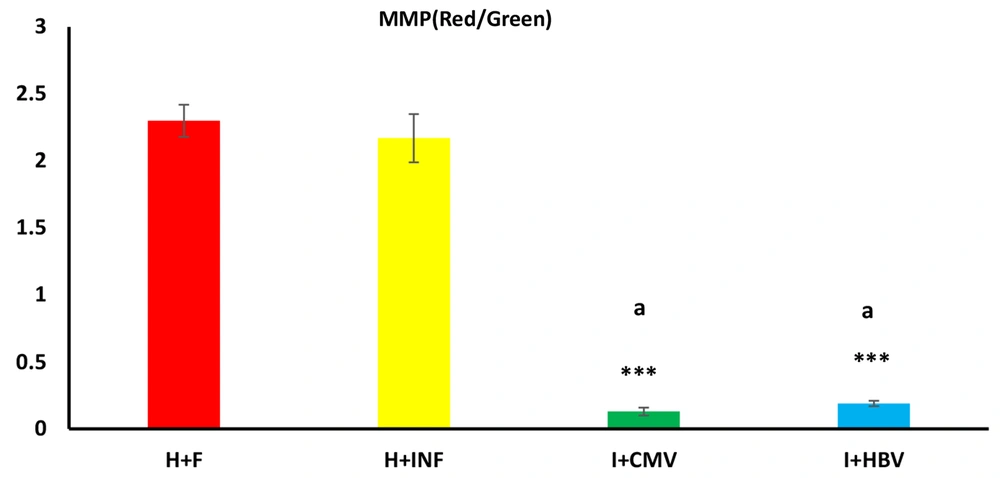
As illustrated in Figure 3, MMP in males with HBV and CMV infections was significantly lower than in the healthy controls and non-infected infertile men (both P < 0.001).
Effects of hepatitis B virus (HBV) and cytomegalovirus (CMV) infections on sperm mitochondrial membrane potential. The mitochondrial membrane potential of sperm is influenced by HBV and CMV infections. This parameter was significantly lower in the infected infertile groups (I + CMV and I + HBV) than in the non-infected infertile (H + INF) and healthy control groups. Data are expressed as mean ± SEM. One-way analysis of variance (ANOVA) and Tukey posthoc test were used to analyze the data. *** P < 0.001 versus the healthy control group; and (a) P < 0.001 versus the non-infected infertile (H + INF) group.
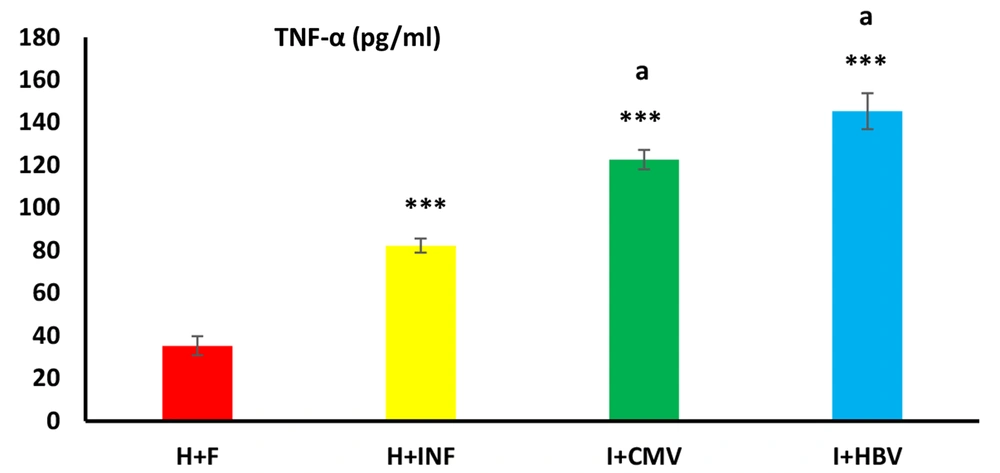
4.3. TNF-α Concentration in Semen Samples
TNF-α, as the studied inflammatory cytokine, was significantly different between groups. This variable significantly increased in the non-infected infertile and infected infertile groups compared to the healthy control people (Figure 4, P < 0.001). TNF-α level in the infected infertile groups (I + CMV and I + HBV) was significantly higher than in the non-infected infertile (H + INF) group (P < 0.001).
Effects of hepatitis B virus (HBV) and cytomegalovirus (CMV) infections on semen TNF-α. The level of this inflammatory cytokine in the HBV- and CMV-infected males was significantly higher than in the non-infected infertile and healthy control groups. Data are expressed as mean ± SEM. One-way analysis of variance (ANOVA) and Tukey posthoc test were used to analyze the data. *** P < 0.001 versus the healthy control group; and (a) P < 0.001 versus the non-infected infertile group.
5. Discussion
This study showed a significant reduction in sperm MMP in infertile males infected with HBV and CMV. The other parameters of sperm, including count, morphology, PR, and PR + NP, were also influenced by HBV and CMV infections. An Investigation in Tehran, Iran, reported that all sperm parameters were significantly impaired in the HBsAg-positive infected men (18). Moreover, another study in Tehran showed a direct correlation between HBV prevalence and male infertility (19). Karamolahi et al. reported that HBV infection significantly decreased the quality of sperm (18). The current results were in agreement with the studies mentioned above. Therefore, it can be concluded that HBV infection affects male fertility by diminishing the quality of sperm parameters.
There have been many studies on the impacts of CMV on male fertility, most of which concluded that the effect of CMV infection on fertility depends on viral load, as a high viral load of CMV in semen samples negatively affected fertility. Mechanistically, studies indicated that a high viral load had a gametotoxic effect by toxicity to immature germ cells and reducing motility (20). A study in Shiraz, Iran, demonstrated that sperm morphology in the high viral load of CMV-positive infertile men reduced compared to the control group (20). Another report revealed CMV infection in infertile men whose sperm motility was affected (21). The current study showed that not only morphology and motility but also sperm count were influenced by CMV infection. Therefore, our results were in line with previous reports that showed consistency between infertility and reduced sperm parameters in infertile males infected with CMV.
The present investigation showed that sperm MMP in infertile males with HBV infection significantly decreased compared to the healthy controls. An in vitro experiment indicated that after the infection of hepatic cells with HBV, the augmented mitochondrion level of hepatitis B X protein (HBX) induced fission and mitophagy, resulting in changed MMP and hepatic cell death. In addition, the increased levels of reactive oxygen species and Ca2+ in the hepatic cells following HBV infection led to the loss of the MMP of hepatocytes and induction of fibrosis (11). Furthermore, in vitro research showed that the exposure of normal sperms to different concentrations of HBV S protein promoted cell death by activating the early phases of apoptosis (22). Consistently, another in vitro research revealed that the exposure of normal sperms to the S protein of HBV decreased sperm motility by inducing MMP loss (23). Therefore, the current in vivo result was in agreement with the above-mentioned in vitro reports. These studies strongly showed that one of the involved mechanisms by which HBV infection reduces the chance of fertility in infected males is the impairment of sperm MMP. There are few studies on the effect of CMV infection on MMP in males. To our knowledge, the current study, for the first time, reported that CMV infection affected male infertility via MMP reduction.
The present study demonstrated that semen TNF-α level in males with HBV and CMV infections was significantly higher than in the healthy control group. Viral infections can cause inflammation, macrophage stimulation, and cytokine release. TNF-α is released in response to acute microbial and viral inflammations (24). Perdichizzi et al. indicated that the increasing level of TNF-α influenced male fertility by impairing sperm function (12). These results may show that HBV and CMV infections impair sperm quality in semen by elevating inflammatory cytokines, such as TNF-α, which eventually leads to infertility. It has been indicated that viral infections cause sperm dysfunction and infertility by inducing oxidative stress in sperm (25). An increase in TNF-α level following viral infections induces oxidative stress by producing reactive oxygen species (11, 24). As a result, HBV and CMV infections increase ROS and target MMP, causing male infertility.
The number of volunteers who participated in the study and followed the rules was the main limitation of this study. Our findings strongly suggested the screening of viral HBV and CMV infections in infertile males. In other words, the lower fertility chance of infertile males with HBV and CMV infection in response to in vitro fertilization or other treatments is due to the decline in all sperm parameters (26). Therefore, screening for HBV and CMV infections has to be considered a must-to-do test for all infertile males.
5.1. Conclusions
The findings of the present study showed that sperm parameters and fertility were impaired by HBV and CMV infections. These viruses decrease the fertility rate in infected men by reducing the MMP of sperm.