1. Background
The severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) is a positive-stranded RNA coronavirus that belongs to the Coronaviridae family and is responsible for causing devastating COVID-19 pandemic with millions of deaths worldwide (1). The genome consists of spike (S), envelope (E), nucleocapsid (N), and membrane (M) proteins. During the SARS-CoV-2 course of infection, the spike glycoproteins bind to angiotensin-converting enzyme 2 (ACE2) across the alveolar type 2 (AT2) epithelial progenitor cells and modulate multiple biochemical pathways to favor the pathogenesis of SARS-CoV-2 (2). To date, most COVID-19 vaccine strategies aim to stimulate spike-specific antibodies with the ultimate goal of blocking the early-stage infection of SARS-CoV-2 (3). According to the World Health Organization, 63 candidate vaccines are in human clinical trials, and > 172 vaccine candidates are at preclinical stages (4). Among SARS-CoV-2 vaccines at the developmental stage, only Gam-COVID-Vac and Sputnik V utilized heterologous (adenovirus 5 and adenovirus 26) prime-boost recombinant adenovirus approach given 21 days apart (to overcome any pre-existing adenovirus immunity), responsibly provoking humoral and cellular immune responses among 98% and 100% of volunteers, respectively (5, 6).
Post-intramuscular injection of Sputnik V helps the replication-deficient Ad26 and Ad5 penetrate host cells and deliver recombinant DNA into the nucleus to stimulate transcription via synthesizing mRNAs and translating spike proteins that migrate toward the cell surface. The vaccinated cells may break spike proteins into fragments. Afterward, protruding spikes, recognized by the immune system, induce strong immune responses to kill the vaccinated cell. However, the cell debris containing spike proteins or fragments is taken up by antigen-presenting cells (APC), which present spike protein fragments on the cell surface. The B cells are activated upon encounter with helper T cells and start proliferation to generate antibodies against spike proteins. The APC can also activate killer T lymphocytes to destroy SARS-CoV-2 infected cells displaying spike protein fragments on surfaces (7).
A phase III clinical trial conducted by the Gamaleya Research Institute, Russia, on 19,866 volunteers who received Sputnik V revealed strong efficacy, immunogenicity, and safety results. The efficacy results were 91.6%, with no serious adverse events (8, 9). Among vaccinated volunteers with a history of wild-type SARS-CoV-2 infection, the virus-neutralizing antibody levels were 1.3 - 1.5 times higher compared to others (6). The levels of neutralizing antibodies are significantly crucial in the context of SARS-CoV-2 vaccines. Reliable quantification of the antibody responses is critical for estimating the time of protection or possible vaccine-related failures. Depending upon the SARS-CoV-2 global burden of continuously increasing COVID-19, there is a dire need for mass vaccination worldwide. In Pakistan, due to several healthcare challenges, identifying neutralizing antibodies against SARS-CoV-2 might be crucial in formulating new vaccination strategies to curtail unprecedented COVID-19 epidemics. To date, 0.8 million people have been infected with COVID-19 in Pakistan, causing deaths in 16,999 individuals. As of 21 April 2021, 1,548,714 vaccine doses have been administered to the general public (10).
2. Objectives
We aimed to evaluate SARS-CoV-2 spike antibody levels among Sputnik V, Sinopharm, and SinoVac vaccinated groups of individuals in the Pakistani population.
3. Methods
A cross-sectional study was conducted on 2000 participants enrolled for Sputnik V (Gamaleya National Research Center, Russia) vaccination after the first dose of Ad26-based vaccine or Sinopharm and SinoVac COVID-19 vaccines to investigate the SARS-CoV-2 spike antibody response to vaccination in the Pakistani population. From 1200 anti-SARS-CoV-2 real-time PCR negative cases, respective clinical samples were obtained on the 21st day by medical experts at the Islamabad Diagnostic Center, Islamabad, Pakistan. Patient written consent was obtained prior to examination. The antibody quantitation of SARS-CoV-2 spike protein was based upon the double-antigen sandwich assay principle using electro-chemiluminescence immunoassay (ECLIA) (Elecsys # 09289267190 Roche, USA). The samples were incubated with biotinylated and ruthenylated RBD antigens, followed by the addition of streptavidin-coated microparticles. They were transferred onto a measuring compartment, with microparticles captured magnetically over the electrode. The electrochemiluminescence is then induced by providing voltage and subsequently measured by photomultiplier tube. Test kits were placed according to the manufacturer's instructions. The current study was approved by the IDC institutional review board.
4. Results
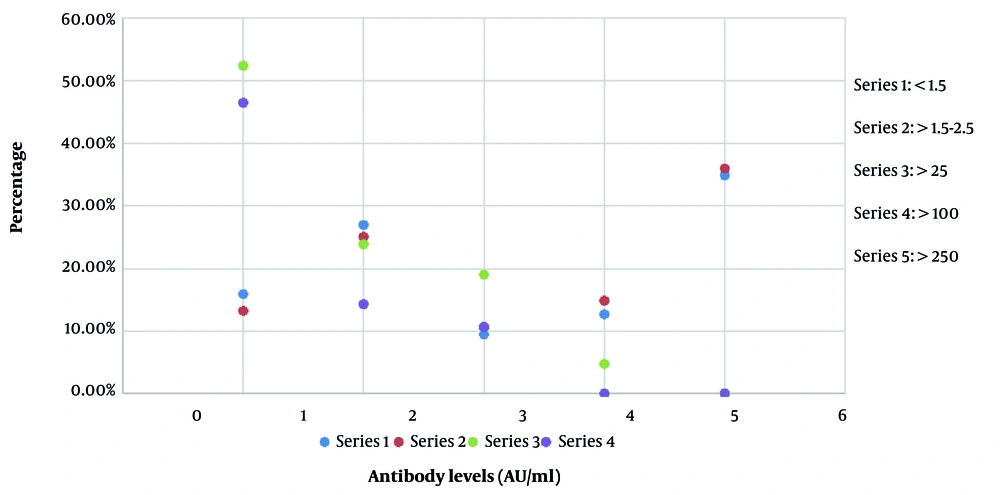
A total of 2000 participants were enrolled in the study. Real-time PCR negative for SARS-CoV-2 RNA (1200) cases were pre-selected from among the participants for evaluation of SARS-CoV-2 spike protein antibody levels 21 days after the first dose administration of Sputnik V, Sinopharm, and SinoVac to 400 subjects of each category, respectively. Among the selected subjects, 61% were males, and 39% were females.SARS-CoV-2 spike protein antibodies > 1.5 AU/mL were detected among 87% of Sputnik V administered, 47.6% of Sinopharm administered, and 25% of SinoVac administered subjects . The mean age was 49 years (range 18 - 72). The ECLIA analysis revealed SARS-CoV-2 spike antibody titers among the enrolled participants. It was shown that > 250 (in arbitrary units, AU/mL) antibody titers occurred only among 36.04% of the participants with Sputnik V administration. Among individuals with > 250 AU/mL antibody levels, 55% had a previous history of COVID-19 infection.
Of the individuals with antibody titers > 100 AU/mL, 14.86% were administered with SputnikV, 4.76% with Sinopharm, and 0.0% with SinoVac. Among SputnikV administered subjects, 10.70% showed antibody titers of > 25 AU/mL, while 19.04% of Sinopharm and 10.71% of SinoVac administered subjects represented antibody titers of > 25 AU/mL. Also, antibody titers of > 1.5 - 2.5 AU/mL were detected in 25.10% of SputnikV administered subjects, 23.80% of Sinopharm administered subjects, and 14.28% of SinoVac administered subjects. Of note, 13.30% of the SputnikV administered participants represented antibody titers of < 1.5 AU/mL, while 52.38% and 46.42% of the Sinopharm and SinoVac administered subjects revealed antibody titers of < 1.5 AU/mL, respectively (Figure 1). Overall, it can be inferred that a high number of subjects showed raised antibody titers against SARS-CoV-2 even before administering the second Sputnik-V vaccine. The sensitivity of ECLIA was 98.8% (95% CI: 98.1 - 99.3%), and the diagnostic assay showed no cross-reactivity against Middle East Respiratory Syndrome Coronavirus (MERS-CoV), common cold, coronavirus HKU1, NL63, 229E, OC43, or other potentially cross-reactive samples.
5. Discussion
In Pakistan, the population exceeds 223.9 million, and the literacy rate is relatively low (11, 12). Viral infections are on their surge (13-18). The vaccine coverage is not successful in Pakistan due to two major reasons, including limited procurement or supply of vaccines and generalized conspiracy theories, which are also prevalent across the globe. In Pakistan, the majority of people are willing to benefit from the vaccine-preventable COVID-19. However, the considerable demand for anti-SARS-CoV-2 vaccine supply from manufacturing companies has significantly affected equitable vaccine distribution in underdeveloped countries. Vaccine hesitancy is an integral, underlying factor linked with the sporadic proliferation of viruses worldwide. Despite the low education level, awareness about vaccination is shown by the fact that people are willing to pay the vaccine's actual price (approximately 70$).
Due to the utmost support of the Pakistan Armed Forces, efficient, innovative lockdown policies, and implementation of standard operating procedures by the National Command Operation Center (NCOC) of Pakistan, the expected number of deaths (80,000 per day), predicted by the Medical Research Council Centre for Global Infectious Disease Analysis, Imperial College London, was efficiently reduced during the first wave of COVID-19 in Pakistan (19). Several countries across the globe and WHO appreciated smart policies implemented by the Government of Pakistan to control COVID-19's first wave in Pakistan. Several multi-variant strains of SARS-CoV-2 are spreading across the nation, and several deaths are being reported from the neighboring country of India. During the need of hour, China and Russia outreached and joined hands in support of vaccine supply under government-government (G/G) or government-business (G/B) schemes to facilitate mass vaccination in Pakistan.
The immune responses to the Sputnik V vaccine were analyzed among 602 healthcare personnel volunteers from Tucumán-Argentina. It is interesting to know that among subjects with prior COVID-19 history of infection, significantly strong immune responses were elicited after a single vaccine dose (20). Similarly, another study was conducted in the United States of America among 110 participants with or without pre-existing SARS-CoV-2 immunity, vaccinated with Pfizer and Moderna COVID-19 vaccines. It was revealed that after the first dose of administration, the antibody titers with pre-existing immunity were around 10 to 45 times as high as those without pre-existing immunity (21).
In contrast to other vaccines with two doses, the Sputnik V is an adenoviral (serotypes 5 and 26) based recombinant DNA vaccine, in which antigen insert is an unmodified full-length S-protein (22). Sinopharm or SinoVac vaccines are based on inactivated viral technologies and are relatively less effective than viral vector or mRNA vaccines against COVID-19 (23). Previously, we evaluated several biomarkers in SARS-CoV-2 positive patients and examined associated high-resolution computed tomography in those patients during the first three waves of COVID-19 in Pakistan (24, 25). Herein, confirmed SARS-CoV-2 real-time PCR negative volunteers of Pakistani origin were pre-selected for the analysis.
Most individuals presented higher anti-SARS-CoV-2 antibody levels of > 250 AU/mL. Among those with > 250 AU/mL antibody titers, 52% showed a previous history of SARS-CoV-2 infections. Generally, the ramp-up of immunity after the first dose is rare among two dosage vaccines. However, Sputnik V interestingly and amazingly aroused ramp-up immunity among most enrolled participants even after the first administration dose. This can further affirm the vaccine's efficacy in the Pakistani population. One of the limitations of our study is that cellular immunity was not evaluated in a clinical laboratory due to a lack of facilities. The current study is important not only for strategic organizations for policy making but also for demonstrating the need to accurately determine SARS-CoV-2 spike antibody levels among a vaccinated group of individuals worldwide.
5.1. Conclusions
Herein, we demonstrated for the first time that compared to Sinopharm and SinoVac, among the Sputnik V vaccinated group, prior to second dose administration, the SARS-CoV-2 spike antibody levels were significantly high, indicating promising results for immunization against COVID-19. It can be inferred that after the second dose of Sputnik V, the SARS-CoV-2 spike antibody levels might further ramp up significantly to immunize against COVID-19. Also, quick immunity with the first dose gives Sputnik V an edge over other vaccines (Sinopharm or SinoVac), which can be helpful when the virus spreads quickly, and many patients become infected before the second dose of other vaccines.