1. Background
Tuberculosis (TB) has been considered a life-threatening disease since Robert Koch discovered it in 1882, and it remains one of the 10 leading causes of death worldwide (1). In 2019, about 10 million active TB infections were diagnosed, with 1.4 million deaths (2). It is caused by a highly contagious bacterium named Mycobacterium tuberculosis (2). Prophylaxis, early detection, and successful treatment are the primary measures for combating TB (3). First-line anti-TB drugs like isoniazid (INH), rifampicin (RIF), pyrazinamide (PZA), streptomycin (STR), and ethambutol (EMB) are mainly used to treat patients with drug-sensitive M. tuberculosis infections (2, 4). However, these medications have severe compliance issues due to their prolonged treatment duration and associated toxicities (5). In addition, latent TB infection and the emergence of resistant strains, particularly multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains, have exacerbated this global problem (2). In contrast, the number of new and updated drugs is insignificant, and just three drugs (bedaquiline, linezolid, and pretomanid) have received FDA approval in the previous 50 years (6). Therefore, we need alternative anti-TB therapeutic candidates with no significant compliance issues and higher effects against drug-sensitive and drug-resistant TB (7).
Natural sources have long been preferred for discovering and developing new antibiotics due to their abundance of structurally diverse, therapeutically active compounds with a diversified functional mechanisms (8, 9). Naphthoquinone is one of the natural compounds with a naphthalene ring. Its derivatives contain several therapeutic properties such as antibacterial, anti-tumoral, anti-leishmanial, anti-helmintic, and anti-fungal activity (10-12). Previous research has shown that naphthoquinones have an anti-mycobacterial effect (13), including activities against MDR and XDR strains (14), reflecting the potential of these compounds for anti-TB drug development. Therefore, we synthesized 17 derivatives based on naphthoquinone and tested them in vitro against M. tuberculosis H37Rv. Among them, we screened a promising compound named methyl-1,4-bis(2-(diethylamino)ethoxy)-2-naphthoate (MDN-6), with excellent anti-TB activity. Further research was carried out on MDN-6 to confirm the qualities required for a potential anti-TB agent.
2. Methods
2.1. MDN-6 Synthesis
We synthesized MDN-6 with 16 other test compounds following the procedure described in the supplementary information. The final purified MDN-6 was collected as a colorless oil with the molecular formula C24H36N2O4 (Figure 1).
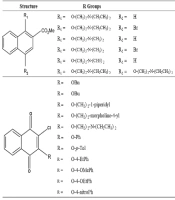
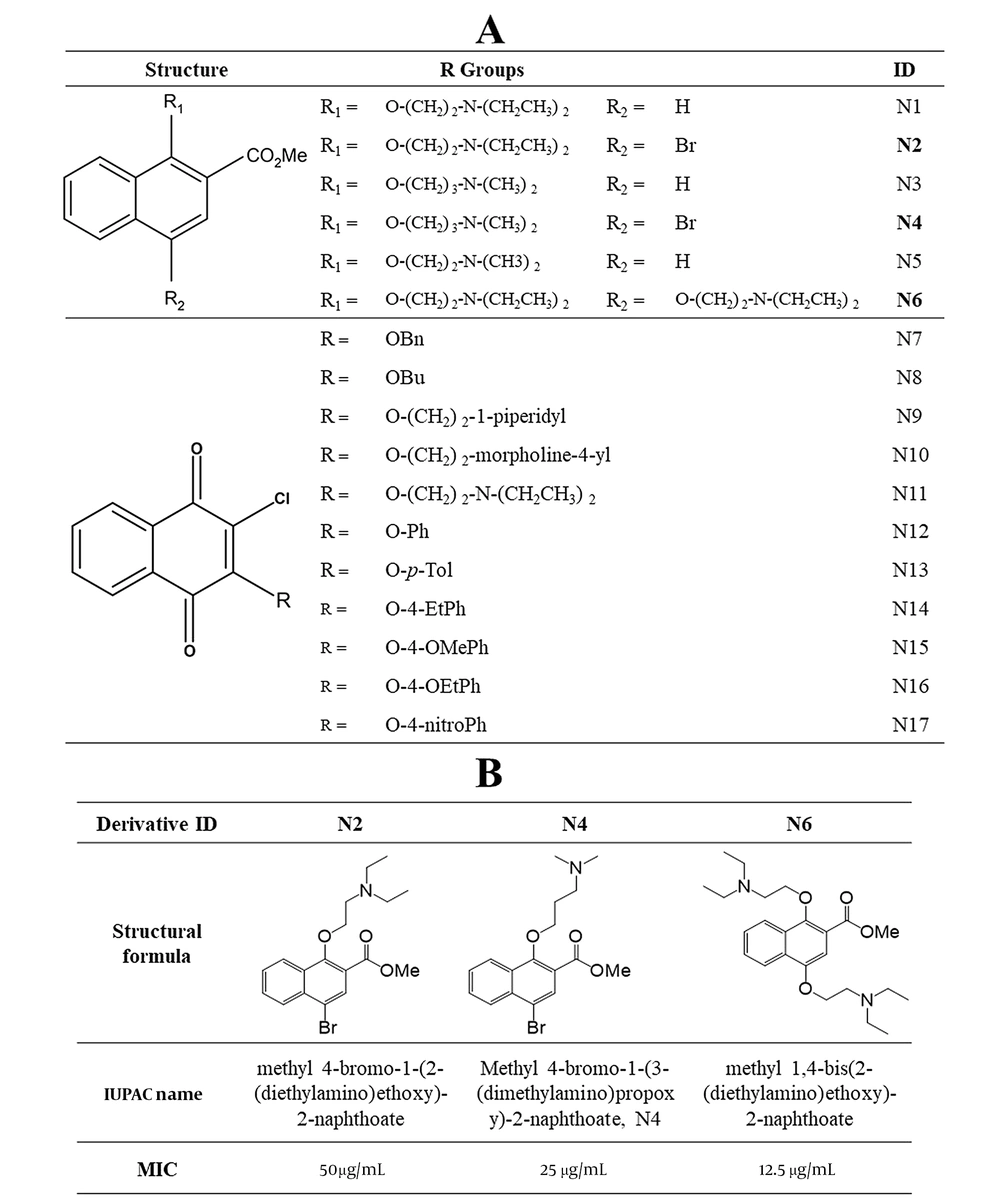
Chemical structures of the synthesized naphthoquinone-based compounds. A, General structures of 17 derivatives screened for anti-TB activity through resazurin test; compounds that showed an inhibitory effect on Mycobacterium tuberculosis H37Rv are shown in bold; B, Chemical structures of potential methyl naphthoate derivatives and their MICs.
2.2. Reference Compounds
We purchased the reference drugs, including EMB, INH, methicillin (METH), PZA, RIF, STR, and vancomycin, from Sigma-Aldrich (USA).
2.3. Resistant Strains of Mycobacterium tuberculosis
This study used 28 drug-resistant strains of M. tuberculosis, including 12 clinically isolated MDR, 12 XDR, and four SDR. Detailed information about these strains is provided in Appendix 1 and Appendix 2.
2.4. Anti-mycobacterial Activity Assessment
The anti-mycobacterial activity of the test compounds was assessed and confirmed using three distinct methodologies. The Alamar Blue assay was used to screen the anti-mycobacterial activities of the test compounds. The luminescent cell viability assay and the CFU counting assay were used to confirm anti-TB and minimum inhibitory activity, respectively. All three assays were performed following the previously published protocols (15, 16), and their detailed descriptions are provided in the supplementary information.
2.5. Synergistic Property Evaluation
The synergistic effect of MDN-6 when used with INH, RIF, STR, and EMB was tested against the drug-sensitive reference strain H37Rv (ATCC 27294) and the drug-resistant strain XDR (KMRC 00203–00197) using a checkerboard titration method according to previous articles (15, 16). The complete method is described in the supplementary information.
2.6. Post-antibiotic Effect
We identified the post-antibiotic effect (PAE) of MDN-6 and the reference drugs against H37Rv (ATCC 27294). The experiment followed a previous protocol (17) with slight modifications. The detailed protocol is supplied in the supplementary information.
2.7. Evaluation of Anti-nontuberculous Mycobacterial Effect
The antimicrobial activity of MDN-6 was evaluated against 27 NTM strains using the broth microdilution technique according to the guidelines of the Clinical and Laboratory Standards Institute (2015). The nontuberculous mycobacterial (NTM) strains and the detailed method of this experiment are provided in the supplementary information.
2.8. Assessment of Activity Against Gram-Positive and Gram-Negative Bacteria
We used a broth microdilution assay following the previous protocol (15, 16) to evaluate the effects of MDN-6 against 24 Gram-positive and Gram-negative bacterial strains. The supplementary information provides all bacterial strains and the complete protocol of this experiment.
2.9. Evaluation of Toxicity in Mouse Model
The toxicity of MDN-6 was evaluated in a mouse model following the guideline of the Korean Safety Evaluation of Drugs (Notification No. 2015-82) at the Soonchunhyang University Animal Facility (LML 20-591). The Institutional Animal Ethics Committee of Soonchunhyang University reviewed and approved the experimental protocol (IACUC SCH21-0026). The toxicity study was conducted following the previous protocol (18). A total of 25 six-week-old female BALB/c mice were purchased for the study and divided into five groups based on the provided treatments: Group-1 (G1) received only vehicle (corn oil), Group-2 (G2) received MDN-6 at 125 mg/kg body weight, Group-3 (G3) received MDN-6 at 250 mg/kg body weight, Group-4 (G4) received MDN-6 at 500 mg/kg body weight, and Group-5 (G5) received MDN-6 at 1000 mg/kg body weight (G5). Finally, the 50% lethal dosage (LD50) was determined by a Questgraph LD50 calculator (AAT Bioquest Inc., USA) (19).
2.10. Statistical Analysis
Every experiment was performed in triplicate. The statistical analysis and graphs were prepared using GraphPad Prism 9 software. The mean and standard deviation (SD) were used to represent all data in graphs. We decided on the significant differences at p < 0.05 (* P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001).
3. Results
3.1. Screening of Naphthoquinone Derivatives as Antitubercular Agents
The primary anti-TB activity screening for 17 different naphthoquinone derivatives was performed with the resazurin microtiter assay (Figure 1A). Among the tested derivatives, methyl naphthoates N2, N4, and N6 showed the inhibitory effects on M. tuberculosis H37Rv and H37Ra. We got a MIC of 50 g/mL for N2, 25 g/mL for N4, and 12.5 g/mL for N6 (Figure 1B). We selected N6 for further study due to its better activity and subsequent lower toxicity among the three compounds. Finally, N6 was named MDN-6 based on its IUPAC name, methyl-1,4-bis(2-(diethylamino)ethoxy)-2-naphthoate.
3.2. Activity of MDN-6 Against Several Drug-Sensitive and Drug-Resistant Mycobacterium tuberculosis Strains
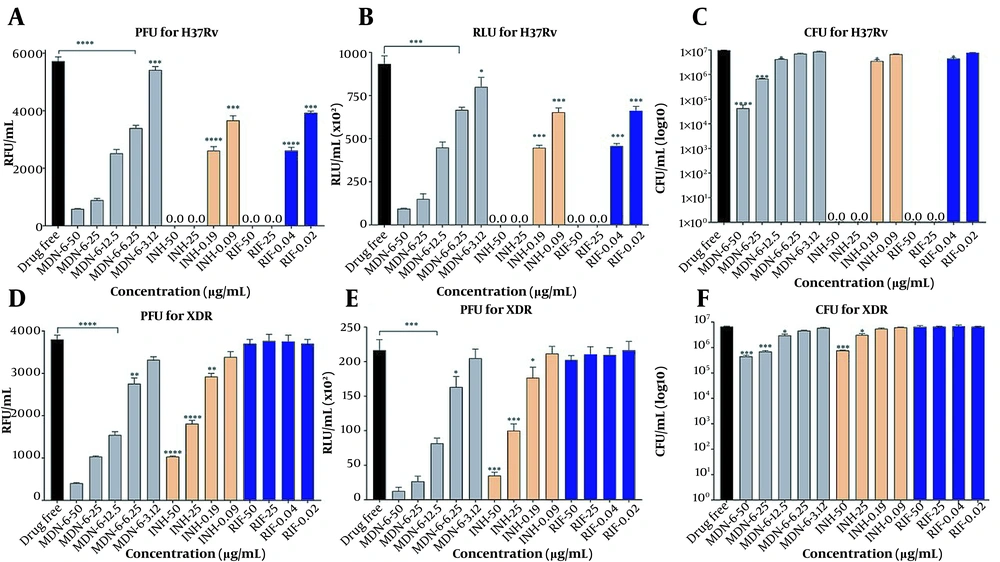
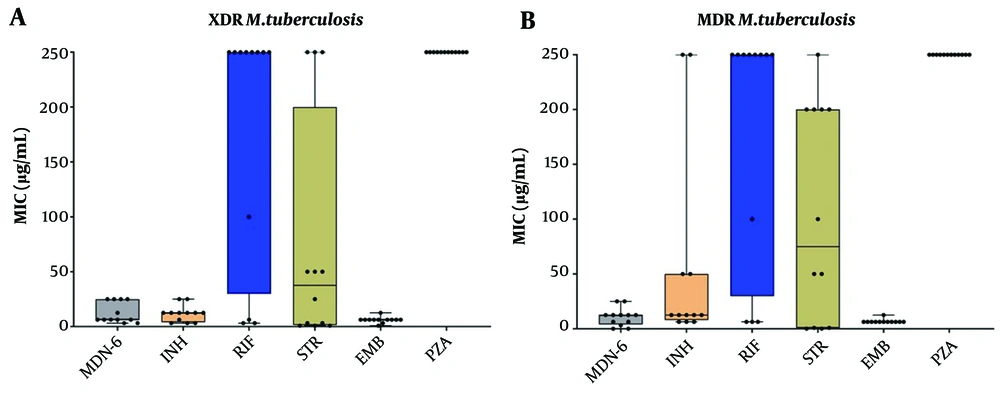
Together with INH and RIF, the anti-TB effect of MDN-6 was initially evaluated against two control strains, H37Rv (ATCC 27294) and XDR (KMRC 00203-00197), using three different experiments (Figure 2). The Resazurin test revealed that MDN-6 had MIC50 values of 12.5 μg/mL against both tested strains, H37Rv and XDR (Figure 2A and B). The luminescent microbial cell viability assay (Figure 2C and D) and CFU counting assay (Figure 2E and F) further confirmed the anti-TB effect of MDN-6, and both of the assays showed similar results at a 12.5 μg/mL concentration. Isoniazid and RIF were more effective at lower concentrations than MDN-6 against H37Rv (Figure 2A, C, and E), though INH and RIF required a greater concentration than MDN-6 for XDR (Figure 2B, D, and F). MDN-6 was next evaluated for antitubercular activity against 24 clinically isolated MDR and XDR strains, and its efficacy was compared to INH, RIF, STR, and PZA. MDN-6 outperformed most of the reference drugs tested against the used MDR and XDR strains, with the MIC values varying from 0.02 to 25 μg/mL (Figure 3 and Appendix 1). MDN-6 also showed significant activity against the tested SDRs as it did against MDR and XDR (Appendix 2).
Antituberculosis activity of MDN-6 and reference drugs against Mycobacterium tuberculosis strains; A, C and E, H37Rv; B, D and F, XDR evaluated by the Alamar blue assay, luminescent microbial cell viability assay, and CFU counting assay. A and B, Resazurin assay results were presented as RFU/mL; C and D, luminescent assay results were presented as RLU/mL; E and F, mycobacterial cell number was presented as CFU/mL.
Antituberculosis effects of MDN-6, isoniazid (INH), rifampicin (RIF), streptomycin (STR), and pyrazinamide (PZA) against clinically isolated drug-resistant Mycobacterium tuberculosis from Korea. A, MIC results of extensively drug-resistant (XDR); and B, MIC results of multidrug-resistant (MDR). The data are given as box plots, with the 25th and 75th percentiles denoted by the bottom and top margins of the box, respectively, and the 50th percentile (median) denoted by the center horizontal line.
3.3. Antibiotic Synergy of MDN-6
MDN-6 had a partial synergistic effect with RIF and STR against XDR (KMRC 00203-00197) M. tuberculosis, with a FICI of 0.562. MDN-6 had an additive effect when combined with INH and EMB (Table 1). MDN-6, on the other hand, had an additive effect with STR and EMB but no effect with INH when tested against H37Rv (ATCC 27294) (Table 1).
| Organism and Antibiotic Combination | FICA | FICB | FIC Index = FICA + FICB | Outcome |
|---|---|---|---|---|
| H37Rv | ||||
| MDN-6 + RIF | FICMDN-6 = 0.5 | FICRIF = 0.062 | 0.562 | Partial synergy |
| MDN-6 + INH | FICMDN-6 = 1 | FICINH = 1 | 2.0 | Indifference |
| MDN-6 + STR | FICMDN-6 = 0.5 | FICSTR = 0.5 | 1 | Additive effect |
| MDN-6 + EMB | FICMDN-6 = 0.5 | FICEMB = 0.5 | 1.0 | Additive effect |
| Extensively drug-resistant | ||||
| MDN-6 + RIF | FICMDN-6 = 0.5 | FICRIF = 0.062 | 0.562 | Partial synergy |
| MDN-6 + INH | FICMDN-6 = 0.5 | FICINH = 0.5 | 1.0 | Additive effect |
| MDN-6 + STR | FICMDN6=0.062 | FICSTR = 0.5 | 0.562 | Partial synergy |
| MDN-6 + EMB | FICMDN-6 = 0.5 | FICEMB = 0.5 | 1.0 | Additive effect |
Abbreviations: RIF, rifampicin; INH, isoniazid; STR, streptomycin; EMB, ethambutol
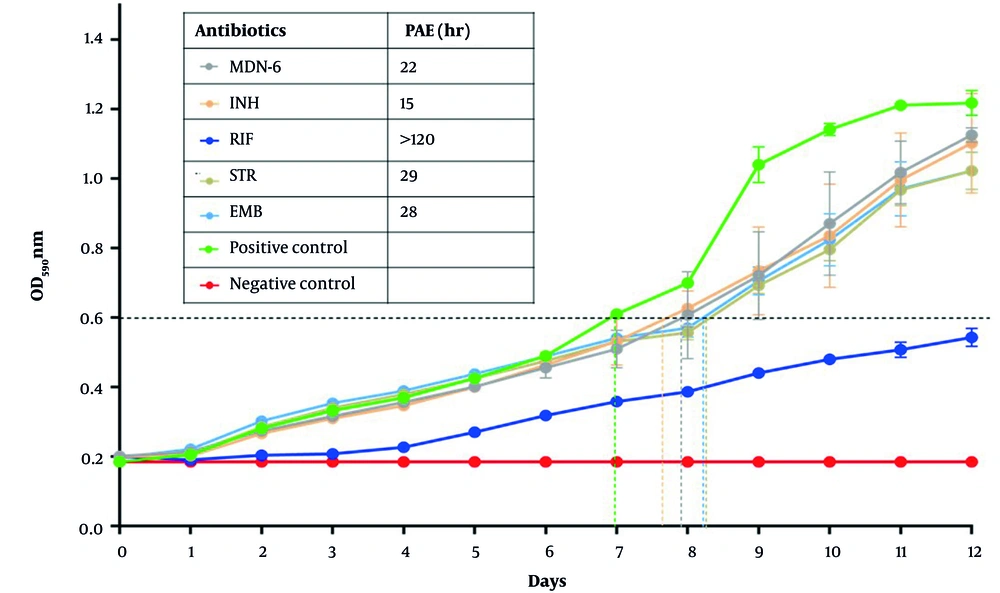
3.4. Post-antibiotic Effect of MDN-6 in Mycobacterium tuberculosis
Post-antibiotic effect is a well-established pharmacodynamic parameter used to test if an antibiotic may delay bacterial growth after antibiotic withdrawal from culture. The PAEs of MDN-6 and first-line control medications were determined using OD600. MDN-6 had a longer PAE value of 22 h than INH (15 h) (Figure 4). However, PAE was longer in STR and EMB (29 and 28 h, respectively) than in MDN-6, and RIF showed the longest PAE value of 144 h (Figure 4).
3.5. Activity of MDN-6 Against Nontuberculous Mycobacteria
MDN-6 and the reference drugs INH, RIF, and STR were evaluated further for their activity against 27 additional NTMs. MDN-6 exhibited an inhibitory effect against M. marinum and M. kansasii with identical MIC values of 12.5 g/mL, but not against the other 25 NTMs (Appendix 3). On the other hand, INH, RIF, and STR showed various effects on the tested NTM strains (Appendix 3).
3.6. Activity of MDN-6 Against Gram-Positive and Gram-Negative Bacteria
MDN-6 was also tested against 17 Gram-negative bacteria and seven Gram-positive bacteria. Up to the highest tested dose (0.02 - 200 μg/mL), MDN-6 did not show an inhibitory effect against any used bacteria (Appendix 4). However, RIF was substantially active against bacterial strains at various concentrations (Appendix 4).
3.7. Acute Oral Toxicity of MDN-6
Mice toxicology study showed no death and abnormal symptoms in the control group, or the group treated with 125 mg/kg of MDN-6 at 30 minutes, one hour, two hours, four hours, and one to 14 days after administration (Appendix 5). However, one animal died in the MDN-6 250 mg/kg treatment group, and all five died in the 500 mg/kg and 1000 mg/kg administration groups, respectively (Appendix 5). Based on the data, we got 279.1 mg/kg as the LD50 of MDN-6 for female BALB/c mice (Appendix 6) using the Questgraph LD50 calculator (AAT Bioquest Inc., USA). After the drug administration, no adverse effect was observed in body weight between the non-treated and treated groups (125 mg/kg and 250 mg/kg) (Appendix 7). The observed mortality and clinical signs after vehicle or drug administration are provided in Appendix 8.
4. Discussion
The one-quarter population of the world is estimated to have a latent TB infection, and roughly 5% of the approximately 10 million TB infections yearly are MDR TB (2). There are around 180 TB deaths daily, and new drug-resistant TB variants are evolving at an alarming rate (2). Around half a million infected individuals demonstrated resistance to at least two first-line antitubercular agents (20). As a result, new and more effective antibiotics capable of killing these lethal resistant bacteria are required immediately. This paper examined the anti-TB activity of 17 naphthoquinone derivatives from a chemical library (Figure 1 and supplementary materials). MDN-6, one of the tested compounds, had significant effectiveness against H37Rv and H37Ra in preliminary testing. As a result, it was chosen for more advanced investigations to assess its drug development potential.
With MIC values ranging from 0.02 to 25 μg/mL, MDN-6 showed significant activity against the control XDR, four single drug-resistant, and 24 additional clinical MDR and XDR TB strains. Previous investigations have revealed that naphthoquinone derivatives had similar effectiveness against diverse drug-resistant clinical isolates of M. tuberculosis, which is consistent with our results (21, 22). Additionally, we performed the chequerboard synergy assay to identify whether MDN-6 had any synergistic efficacy with the core anti-TB drugs or not, as it was reported that the synergistic activity of the new drug-current drug combination could help to reduce the treatment duration and restore the lost antibiotic activity of the drug against the respective resistant strain (23, 24).
MDN-6 had a partially synergistic effect with RIF and STR against XDR M. tuberculosis in a chequerboard synergy assay. These results suggest that DNF-6 could be combined with RIF and STR to treat drug-resistant TB, reduce the treatment time, and abolish latent TB. However, the mechanism behind the partial synergistic and additive activity of MDN-6 with the core anti-TB drugs against XDR is not precise yet, requiring further study. Overall, MDN-6 demonstrated considerable effect against 24 drug-resistant MDR and XDR and four SDR strains of M. tuberculosis, with the intracellular killing activity and a partial synergistic property. All the information suggests that it might be an ideal alternative therapy option for existing and emerging drug-resistant TB infections.
The PAE of an antimicrobial compound is essential for determining the appropriate gap between drug intake, and an extended PAE enables a longer time window between doses while maintaining antimicrobial efficacy (25). MDN-6 had a longer PAE (22 h) than INH (15 h), demonstrating its capacity to inhibit mycobacterial multiplication by about one doubling time (24 h) (26, 27). This result suggests that MDN-6 can be used at a wider dosing interval without losing its therapeutic efficacy. Thus, MDN-6 merits further study for drug development. We further evaluated the activity of MDN-6 against 27 NTM strains, but it did not show any significant effect against most of the NTM strains, except against M. marinum and M. kansasii. Previous studies have reported that M. tuberculosis, M. marinum, and M. kansasii share a close phylogenetic position with similar pulmonary infection locations and pathogenesis patterns (28, 29), which could be the reason behind the activity of MDN-6 against these three bacteria.
In the MIC test with 24 Gram-positive and Gram-negative bacteria, other antibiotics showed an overall growth inhibitory effect, whereas MDN-6 did not, indicating that the antibacterial effect of MDN-6 is mycobacterium-specific. We also assessed the toxicity of MDN-6 in a mice model (female BALB/c mice). The 279.1 mg/kg LD50 value of MDN-6 is comparable to the LD50 value of previously reported anti-TB compounds (30). Overall, the LD50 value of MDN-6 is not completely non-toxic but it is better than many approved anti-TB drugs such as capreomycin (LD50 = 250 mg/kg), clarithromycin (LD50 = 184 mg/kg), moxifloxacin (LD50 = 105 - 130 mg/kg), and isoniazid (LD50 = 151 mg/kg) (31). In addition, other toxicity tests, such as long-term repeated toxicity tests and genotoxicity tests for MDN-6, are further needed.
4.1. Conclusions
In conclusion, MDN-6 can be a reliable alternative treatment for drug-resistant TB infection due to its significant anti-TB activity against various drug-resistant Mycobacterium tuberculosis strains and its partial synergistic effect. However, additional in-depth investigations on the action mechanism of MDN-6, animal effectiveness, and toxicity are required.