1. Background
Invasive fungal infections are associated with high morbidity and mortality in patients with prolonged hospital stay, and recipients of hematopoietic stem cell and solid organ transplant (1, 2). Voriconazole is a wide-spectrum triazole medication recommended for the treatment of potentially life-threatening fungal infections (3, 4). It is considered as the therapy of choice for invasive aspergillosis (5), and a valid alternative for treating disseminated candidiasis (6) and other fungal infections caused by Fusarium and Scedosporium species (7, 8).
The mode of action for voriconazole is binding in the active-site cavity of cytochrome P450 sterol 14α-demethylase enzyme and inhibiting the synthesis of ergosterol, cell-membrane, and fungal growth (9). The oral bioavailability, binding rate to plasma proteins, and tissue penetration of this agent are > 90%, 58%, and 2 to 4.6 L/kg, respectively. The elimination half-life of voriconazole is approximately 6 hours with < 2% of the drug excreted unchanged in the urine (10). Voriconazole plasma levels are highly variable, attributed to several factors, which influence its steady-state blood concentration (11, 12).
Genetic polymorphisms of cytochrome P450, mainly CYP2C19 enzyme, which effect the metabolism of voriconazole, patients’ age, drug-drug interaction, specific clinical conditions such as organ failures and liver function abnormalities, lead to substantial inter and intra-individual variability of voriconazole plasma trough concentrations in clinical practice (13-15). These variations might be associated with decreased efficacy or increased toxicity (16). Low voriconazole levels (< 1.0 µg/mL) are associated with therapeutic failure and elevated levels (≥ 5.5 µg/mL) are correlated with an increased risk of adverse events (hepatotoxicity, hallucination, visual disturbance, and skin rash) (17-19).
Therapeutic drug monitoring (TDM) is a potentially useful tool for preventing toxicities related to voriconazole by clinicians (20). Different methods such as gas chromatography, mass spectrometry, and ultra-performance liquid chromatography have been described for the evaluation of voriconazole concentrations (21). Many studies have applied high-performance liquid chromatography (HPLC) as an accurate technique, to quantify voriconazole plasma levels with acceptable sensitivity (22, 23). However, given the high price of the equipment for HPLC, this method is not widely used for voriconazole measurement in routine clinical laboratories. Bioassay has also been used as a microbiologic technique, which is reliable and easy to perform (24).
2. Objectives
This study aimed at describing a simple and valid agar well diffusion bioassay for quantification of voriconazole plasma levels and comparing the accuracy and precision of this method with HPLC in a group of voriconazole-treated patients.
3. Methods
3.1. Preparation of Standards and Control Samples
Reference standard of voriconazole (purity ≥ 98%) was purchased from Sigma (Sigma-Aldrich, Germany). Two separate stock solutions were prepared by dissolving voriconazole in dimethyl sulfoxide (DMSO; Sigma-Aldrich, Germany) to obtain the concentration of the drug (1600 µg/mL). Calibration standard samples were prepared by mixing 990 µL of human serum (Commercial human male AB Plasma, Sigma-Aldrich) with 10 µL aliquots of the first stock solution in a series of 2-fold dilutions to give the final concentrations of 16, 8.0, 4.0, 2.0, 1.0, 0.5, 0.25, and 0.125 µg/mL. Quality control samples, containing 0.25, 0.5, 1.0, 2.0, 4.0, and 8.0 µg/mL of voriconazole were prepared in the same way using the second stock solution. For evaluating the selectivity and specificity of methods, other antifungals and antibacterial drugs consisting of fluconazole, itraconazole, caspofungin (Sigma-Aldrich), imipenem, amoxicillin/clavulanic acid, ciprofloxacin, ceftazidime, and gentamicin (Oxoid SA, Madrid, Spain) were used (25).
3.2. Clinical Performance and Concordance Between High-Performance Liquid Chromatography and Bioassay
Clinical utility of bioassay was evaluated by comparing the measure of voriconazole plasma levels in 180 samples from 60 patients receiving voriconazole treatment, using both HPLC and bioassay methods. For oral administration, loading dose of 400 mg on the first day, followed by 200 mg twice daily, for patients with weight of ≥ 40 kg, and 200 mg followed by 100 mg twice daily for patients with weight of ≤ 40 kg were prescribed. For intravenous therapy, 2 loading doses of 6 mg/kg/12-hours on the first day, followed by 4 mg/kg/12-hours were used for all patients, according to the specific guideline (26). The patients receiving combination antifungal therapy were excluded from the study. Blood samples (3 mL EDTA) were drawn 30 minutes prior to administration of the next voriconazole dose (20). Plasma was stored at -70°C for further analysis.
3.3. Bioassay of Voriconazole
The biological activity of voriconazole in serum samples was measured by a diffusion assay. A clinically isolated voriconazole susceptible strain (MIC ≤ 0.015 µg/mL) of Candida kefyr, identified by API 20C AUX system (bioMerieux Vitek, Hazelwood, Mo.) and restriction fragment length polymorphism, was used as a test organism. The organism was cultured on sabouraud dextrose agar (MERCK, Germany) and colonies were suspended in 3.0 mL of sterile water. The turbidity was adjusted to 1 McFarland standard equivalent to 1 - 5 × 108 CFU/mL (optical density at 530 nm between 0.23 and 0.27). A broth medium containing 6.7 g yeast nitrogen base (YNB, Difco Becton Dickinson), 30 g glucose (Sigma-Aldrich Chemie, Germany), and 5.9 g trisodium citrate (Sigma-Aldrich Chemie, Germany) was prepared in 1000 mL distilled water, and pH was adjusted to 7.0, and the solution was filtered by a syringe filter (Jet Biofil, China).
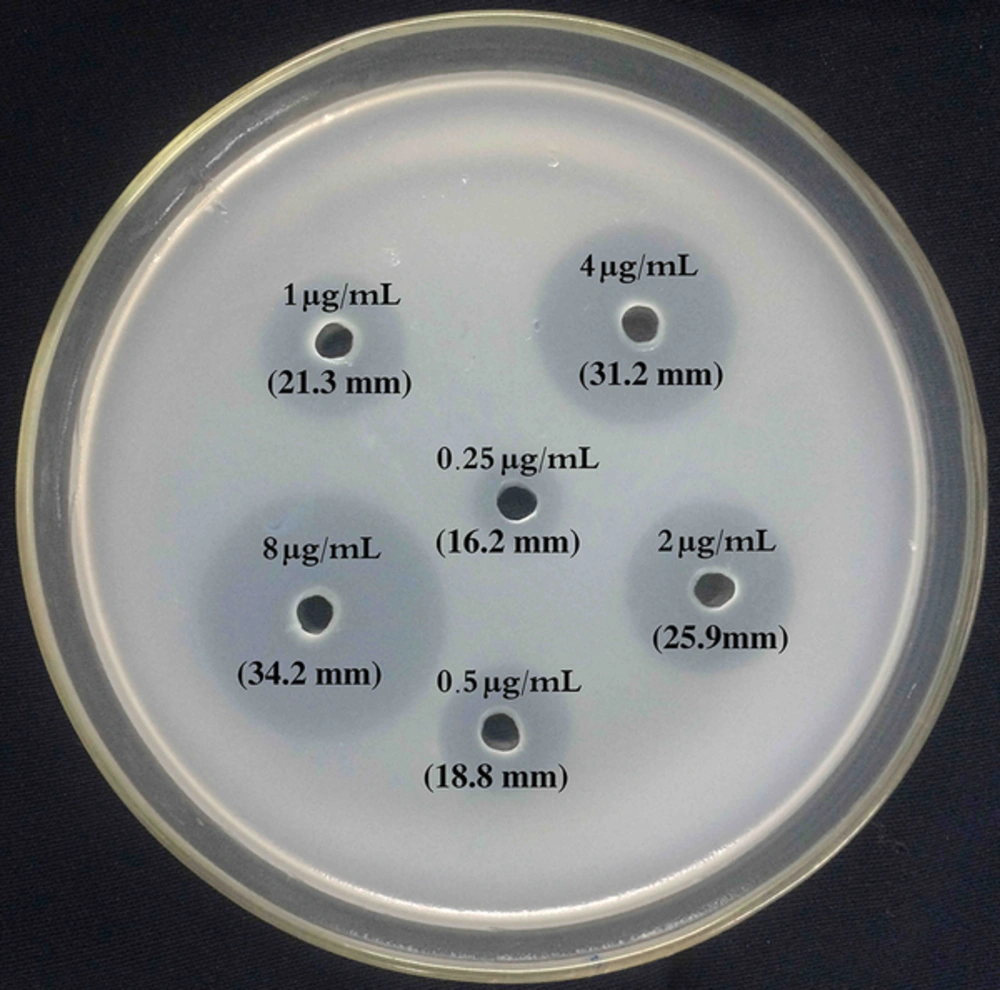
The agar medium (MERCK, Germany) was made (15 g/L) and autoclaved for 15 minutes at 121°C. After cooling down to 48°C, 45 mL of agar, 5 mL of filtered broth medium, and 1 mL of adjusted C. kefyr suspension were gently mixed by inversion and poured in sterile a 150 mm × 150 mm petri dish. The agar was left to solidify at room temperature for 30 to 45 minutes. In addition, 6 round wells (5 mm diameter) were bored using sterile crock borer (Figure 1). Twenty-five microliters of each standard, control, and patients’ plasma were pipetted to each well of the plate, allowed to diffuse through the agar at room temperature for 30 minutes, and incubated at 37°C for 24 hours. The inhibition zones were quantified by measuring the zone diameter using a metric caliper micrometer (Glattbach, Germany) with precision of 0.1 mm. Measurements were done in duplicates by 2 different investigators. All test runs were repeated 3 times daily on 3 different days.
3.4. High-Performance Liquid Chromatography Assay of Voriconazole
3.4.1. Sample Preparation
Serial dilutions of calibration standard and quality control in human serum and serum samples of patients were processed as follows: 200 µL ice-cold acetonitrile (Merck, Darmstadt, Germany) was added to the 200 µL-aliquots of each sample for protein precipitation. These mixtures were vortex-mixed for 30 seconds and centrifuged at 13,800 rpm and 25°C for 15 minutes. Supernatants were transferred to injection vials. For each run, 60 µL of supernatant was injected to liquid chromatography (27).
3.4.2. Chromatographic Conditions
The HPLC method was described by Cendejas-Bueno et al. (27). Reversed- phase (RP-18) HPLC analyses were performed using a Knauer analytical HPLC with a K- 1001 pump (Knauer, Berlin, Germany) and a variable wavelength ultraviolet spectrophotometric detector (Knauer PDA 2800) set at wavelength of 262 nm. The separation was carried out by a 125-mm × 4.60-mm inside-diameter reverse-phase column (Nucleodur 100-5 C18 ec), maintained at room temperature (25°C). The mobile phase consisted of deionized water (Millipore Milli-Q system, Watford, UK) and HPLC-grade acetonitrile (≥ 99.9%, Merck, Darmstadt, Germany) at 60/40 (vol/vol) proportions. The total run time was 9 minutes at a flow rate of 0.4 mL/minute. The EZChrom Elite Software was used to control the HPLC system, monitor the output signals, and plot the chromatograms.
3.5. Validation Procedure for Bioassay and High-Performance Liquid Chromatography
3.5.1. Linearity Assessments
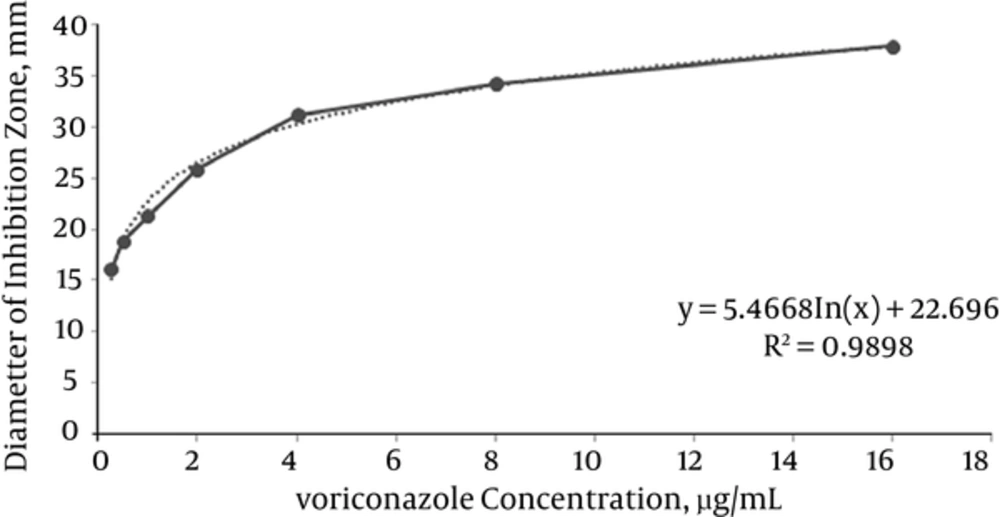
To evaluate the linearity of the bioassay using 8 calibration standard samples (ranging from 0.125 to 16.0 µg/mL), a standard curve for the concentrations of voriconazole (µg/mL) versus zones of inhibition diameter (mm) was plotted. For HPLC, the standard curve was constructed by plotting the peak areas measured by HPLC against the concentrations of the 8 standards. Linearity assessments for both methods were performed by linear regression analysis and were validated with a correlation coefficient of (R2) ≥ 0.99.
3.5.2. Accuracy and Precision
The inter-run and intra-run accuracy and precision (expressed as relative error and coefficient of variation, respectively) of HPLC and bioassay were determined by analyzing 6 quality control samples (0.25, 0.5, 1.0, 2.0, 4.0, and 8.0 µg/mL). For the intra-run assay (within-day), the voriconazole concentrations in quality control samples were measured in triplicates on the same day and for the inter-run assay (between-day), quality control samples were processed during 3 non-consecutive days. The accuracy should have been within ± 15% deviation from the nominal values, precision within ± 15% of coefficient of variation, and the lower limit of quantification, should not have exceeded 20% of the coefficient of variation, as recommended by the U.S. food and drug administration (FDA) guide lines (28).
3.5.3. Analytical Recovery
Percentage recovery of voriconazole from plasma was calculated by comparing the peak area of 4 extracted serum samples spiked with voriconazole (0.5, 1.0, 2.0, and 4.0 µg/mL) with 4 aqueous solutions of voriconazole of the same concentrations using the following formula: measured concentration in plasma/measured concentration in water × 100 (28).
3.5.4. Selectivity and Specificity
Selectivity was tested using 6 independent batches of human plasma samples in order to check for the presence of potential interferences of endogenous substances. Specificity was indicated by the absence of interference peaks at same retention times of voriconazole for HPLC and no zone of inhibition in bioassay results. The specificity of both methods was determined by analyzing various blank samples and samples from patients, who had received commonly used anti-bacterials (imipenem, amoxicillin/clavulanic acid, ciprofloxacin, ceftazidime, and gentamicin) or antifungals (fluconazole, itraconazole, and caspofungin).
3.6. Ethical Consideration
This study was carried out in accordance with the guidelines of the declaration of Helsinki, as revised in Edinburgh (1975). The study protocol was approved by the ethics committee of Prof. Alborzi clinical microbiology research center (ec-92-6884). Written informed consents were obtained from all patients prior to the blood sampling.
3.7. Statistical Analysis
For calculations, HPLC was considered as the reference method. Linearity assessment for both methods (HPLC and bioassay) was performed by linear regression analysis. The within and between-run variability of the assays were estimated by computing the coefficient of variation and relative error. The agreement between both analytical methods was evaluated using the correlation coefficient.
4. Results
4.1. Bioassay
Regression analysis showed that the standard curve of the bioassay was logarithmic in the range of 0.25 to 16 µg/mL with a correlation coefficient of R2 = 0.989 in all runs (Figures 1 and 2). The limit of detection and lower limit of quantification of the bioassay method were found to be 0.25 µg/mL. The results of the intra and inter-run validation are shown in Table 1. The bioassay method was found to be precise, as the repeated measurements of the control samples (three times daily for three different days) showed the within and between-day accuracies of bioassay method ranged from 1.28% to 9.6% and 2.23% to 10%, and the precisions ranged from 2.08% to 7.25% and 3.65% to 6.99%, respectively. The coefficient of variation and relative error data were in agreement with international recommendations for bio-analytical methods (28).
| Voriconazole Concentration of QC Samples, µg/mL | Within-Day Variability | Between-Day Variability | ||||
|---|---|---|---|---|---|---|
| C, µg/mL | Accuracy RE, % | Precision CV, % | C, µg/mL | Accuracy RE, % | Precision CV, % | |
| HPLC | ||||||
| 0.25 | 0.265 ± 0.013 | 6.00 | 4.90 | 0.261 ± 0.012 | 4.40 | 4.59 |
| 0.5 | 0.526 ± 0.035 | 5.20 | 6.65 | 0.512 ± 0.011 | 2.40 | 2.14 |
| 1.0 | 0.943 ± 0.039 | -5.70 | 4.13 | 0.968 ± 0.030 | -3.20 | 3.51 |
| 2.0 | 2.088 ± 0.087 | 4.40 | 3.02 | 2.099 ± 0.015 | 4.95 | 0.71 |
| 4.0 | 4.060 ± 0.080 | 1.50 | 1.97 | 4.090 ± 0.033 | 2.25 | 0.80 |
| 8.0 | 7.970 ± 0.264 | -0.37 | 0.33 | 8.040 ± 0.049 | 0.50 | 0.60 |
| Bioassay | ||||||
| 0.25 | 0.274 ± 0.015 | 9.60 | 5.47 | 0.275 ± 0.019 | 10.0 | 6.90 |
| 0.5 | 0.538 ± 0.039 | 7.60 | 7.25 | 0.543 ± 0.038 | 8.60 | 6.99 |
| 1.0 | 1.030 ± 0.055 | 3.00 | 5.34 | 1.042 ± 0.061 | 4.20 | 5.85 |
| 2.0 | 2.042 ± 0.113 | 2.10 | 5.53 | 2.080 ± 0.093 | 4.00 | 4.47 |
| 4.0 | 4.193 ± 0.234 | 4.82 | 5.58 | 4.257 ± 0.185 | 6.42 | 4.34 |
| 8.0 | 8.103 ± 0.169 | 1.28 | 2.08 | 8.179 ± 0.299 | 2.23 | 3.65 |
Abbreviations: C, experimental concentration; CV%: coefficient of variation in percent; HPLC, high-performance liquid chromatography; QC, quality control; RE%: relative error in percent; SD, standard deviation.
aThree times daily for three different days.
bValues are expressed as mean ± SD.
4.2. High Performance Liquid Chromatography
High-performance liquid chromatography results demonstrated a linear relationship between peak height ratios and voriconazole concentrations over a range of 0.25 to 16 µg/mL (R2 > 0.99). The limit of detection and the lower limit of quantification by the HPLC method were found to be 0.125 and 0.25 µg/mL, respectively. The mean retention time for voriconazole was 4.5 ± 0.3 minutes. The within and between-day accuracies of HPLC ranged from -0.37% to 6% and -3.2% to 4.95%, while the precisions ranged from 0.33% to 6.65% and 0.6% to 4.59%, respectively (Table 1). The percentage recovery of voriconazole from extracted samples was in the range of 94.7% to 100%, indicating the consistent, precise, and reproducible extraction efficiency of the HPLC as the standard method. Endogenous components of voriconazole-free samples (blank) did not show any interference with peak heights of voriconazole at the retention times. Due to high specificity of the method, no interaction was observed between voriconazole signal, and possible co-administered of antifungal and antibacterial drugs.
4.3. Correlation of Bioassay Results with High-Performance Liquid Chromatography
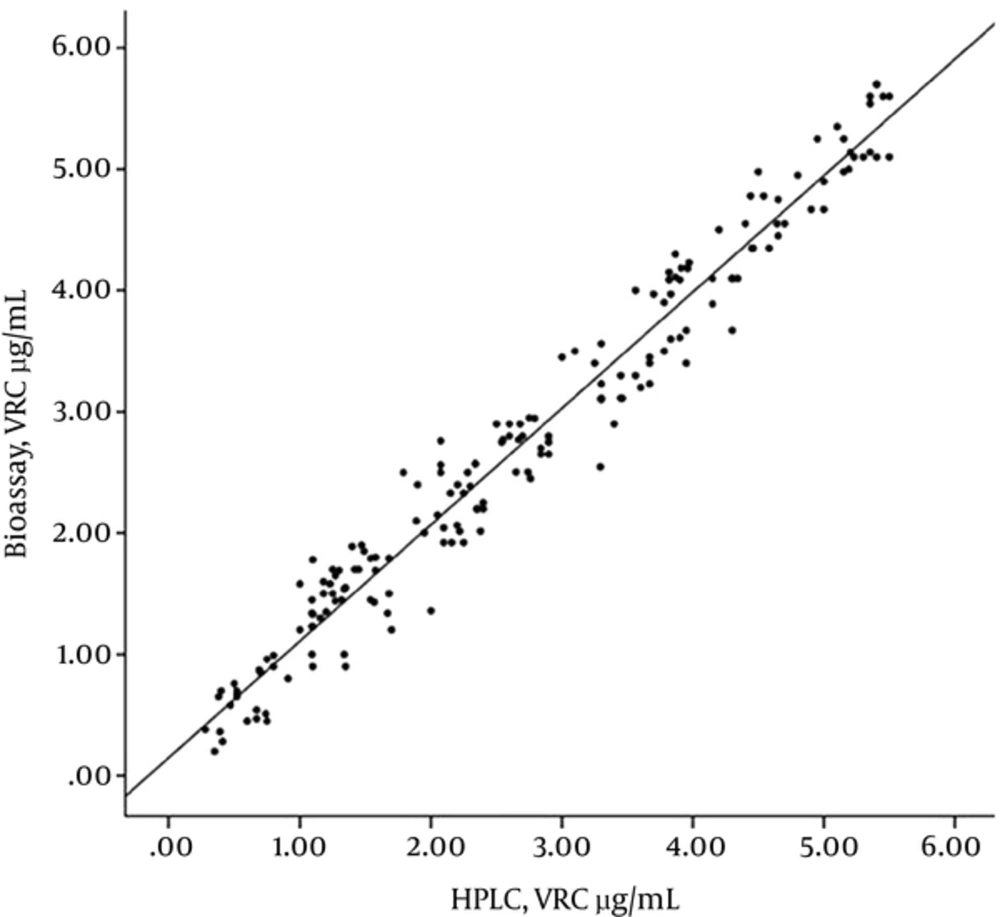
The correlation between voriconazole levels measured by bioassay and HPLC in clinical samples was evaluated in 180 samples from 60 patients (Figure 3). Voriconazole serum concentration of patients ranged from 0.25 µg/mL to 5.41 µg/mL when using HPLC, and from 0.25 µg/mL to 5.71 µg/mL when using the bioassay method. Values below the lower limit of quantification were considered as 0.00 µg/mL. Comparing the results of 2 analytical methods, 31% of the samples showed voriconazole concentration less than 0.25 µg/mL by HPLC and bioassay methods. The concordance between the results of HPLC and bioassay methodologies was assessed, as indicated by the scatterplot in Figure 3. The result of linear regression analysis showed bioassay = 0.961 (HPLC) + 0.148; R2 = 0.965; correlation coefficient = 0.982; n = 180.
5. Discussion
The rate of invasive fungal infections is growing parallel with increasing number of immunocompromised patients (29, 30). Voriconazole, as an effective agent, is currently used for the treatment of invasive aspergillosis (31). Despite initiating an appropriate drug and regimen, inadequate drug exposure at the site of infection might occur due to pharmacokinetic variability of voriconazole (19). Therefore, to improve the efficacy and safety and minimize the risk of adverse events, therapeutic drug monitoring by an accurate and reliable assay is crucial. In this study, a validated simple bioassay method was compared with HPLC for the quantification of voriconazole levels in patients. Linear regression analysis showed an excellent correlation between the 2 analytical methods (correlation coefficient = 0.982).
Based on FDA recommendations, the bioassay has valid criteria for measurement of voriconazole concentrations in biological matrices (28). This method gives the possibility to evaluate potency and monitor the biological activity of voriconazole in patients (32). Voriconazole level in plasma, following the administration of multiple oral or intravenous doses, varies from 1.0 to 5.5 µg/mL (20, 33), which are within the limits in which the bioassay method was linear (0.25 - 16 µg/mL) in this study. Therefore, linearity range obtained by this method effectively covers what is currently believed to be the clinically relevant range for voriconazole concentrations in plasma. Therefore, there was not any limitation of practical applicability for the detection of voriconazole-related toxicity or therapeutic failure by the suggested bioassay method.
In this study, HPLC, as the standard method, was highly specific and no interaction was observed between voriconazole signal and possible co-administered antifungal and antibacterial drugs. One of the practical advantages of the proposed bioassay is being easy to perform with no special equipment and expertise required that might not be available in all clinical microbiology laboratories. Protein precipitation in samples was not used as a pretreatment procedure. Another advantage is a small volume of plasma sample needed to perform this bioassay (75 µL), which is particularly important in the case of pediatric patients, compared to the HPLC method, which uses at least 200 µL of plasma.
The proposed bioassay is slightly different from other microbiological methods described in previous studies (34-36). In this study, a specific clinical isolate of C. kefyr (voriconazole-hyper susceptible strain, MIC ≤ 0.015 µg/mL) was used as the test organism. Previous experiments involving bioanalytical methods have used some types of reference strains provided by American type culture collection (24, 34) or applied a mutant of C. albicans, constructed by targeted deletions of genes in their studies (36). According to the present study, use of in-house clinical isolates of C. kefyr susceptible to voriconazole, which provided well-defined and symmetric zones of growth inhibition, is suitable as the test organism. It is worth mentioning that the bioassay has some limitations.
Analytical methods such as HPLC exclusively rely on the assessment of compounds with a predefined chemical structure. It could discriminate the parent compound from related metabolites. The bioassay is unable to identify the active metabolites form of drugs. The other limitation of the bioassay method is combination of antifungal therapy by patients, which may effect the regular inhibition zone of the growth. Therefore, a clear interpretation of the voriconazole concentration is impossible in these patients. The third limitation is the time for conducting the test; the HPLC method is a valuable option when the results are needed quickly (3 hours), but in bioassay, results need longer analytical time, approximately 24 hours. Unfortunately, in many countries, HPLC equipment is very expensive and blood voriconazole concentrations are not evaluated for patients.
The current study evaluated the bioassay and HPLC method and revealed a good concordance between them for the measurements of voriconazole plasma levels in 180 samples from 60 patients. The proposed bioassay with sufficient accuracy and precision may be a valid alternative tool to HPLC in clinical laboratories without specialized facilities.