1. Introduction
Two species of Leishmania are responsible for cutaneous Leishmaniasis infections in Iran. Leishmania major and L. tropica are causative agents of zoonotic cutaneous leishmaniasis (ZCL) and anthroponotic cutaneous leishmaniasis (ACL), respectively. Both forms of this disease exist in Iran. Zoonotic cutaneous leishmaniasis is an endemic disease in 4 major regions of Iran: central parts (Isfahan province), north Iran (Semnan province), southwest Iran (Khozestan and Ilam provinces), and south Iran (Fars, Bushehr and Hormozgan provinces) (1, 2). Anthroponotic cutaneous leishmaniasis is endemic in Tehran (capital city of Iran), Shiraz, Mashhad, and Kerman (3). It was reported that the 45 isolates of Leishmania from different geographical areas of Iran were suspected of cutaneous leishmaniasis (4). Every year it was reported that at least 20 000 cases of cutaneous leishmaniasis are in Iran (5).
For many years, Khorasan province has a lot of foci of ACL and ZCL (1, 6). Leishmania tropica in most common species that has been reported from Mashhad (1, 3), Khaf (5), Torbat-e Heydarieh (7), Kharve (8), Taybad (9), and Torghabeh - Shandiz (10). Sarakhs (11), Dargaz (unpublished data), Turkemen sahara (12), and Sabzevar (2) were introduced as L. major focuses.
2. Objectives
The aim of this study was to identify the presence of different species of Leishmania in most endemic areas of CL in Khorasan province including Gonabad, Bardaskan, Kashmar. There is no previous study regarding the leishmaniasis epidemiology in these cities.
3. Methods
3.1. Study Area
Kashmar is located near the river Sish Taraz in the western part of the province, 217 kilometers away from Mashhad; the capital of Khorasan province, with a population of 81,527 inhabitants. Gonabad, located at 287 kilometers km distinct at the south of Mashed has a population of 36 367 in habitats. The distance from Bardaskan to Mashhad is 288 kilometers at the margin of the north part of the salt desert (https://en.wikipedia.org/wiki/Kashmar). These cities have warm and dry climates with several seasonal rivers but no permanent river, as shown in Figure 1.
3.2. Sampling
In this cross-sectional study, people who had at least 1 skin ulcer suspected to CL were selected and introduced to clinical laboratories of the health center of Gonabad, Bardaskan, and Kashmar cities for direct examination. Prior to collecting, demographic and clinical information was obtained by a questionnaire. An informed consent form was obtained from each participant.
3.3. Parasitological Examination
To prepare direct Giemsa stained smears, samples were obtained by scraping from the center and edge of each skin lesion using a sterile scalpel (for each patient 2 smears). After air-drying, the slides were fixed in methanol, and stained with Giemsa (catalogue number: 1092040500). An expert microscopist examined each smear 2 times. Some scraping wound was retained in separately labeled containers in 70% ethanol for molecular technique.
3.4. Reference Strains
Strains MRHO/IR/75/ER of L. major and the strain MHOM/IR/01/yaza of L. tropica were used in this study.
3.5. DNA Extraction
DNA extraction from tissue lesions was processed using the Ge Net Bio Kit (Korea), according to the manufacturer's protocol. The quantification and quality control of the DNA extraction procedures were performed using a nano spectrophotometer (NanoDrop 1000, Thermo Fisher Scientific). To avoid sample contamination, reactions were performed in appropriate places, following the good practice of laboratories.
3.6. PCR for Leishmania kDNA
A conventional PCR was used for the detection of Leishmania species and specific primers for kDNA were derived as forward (5’-TCGCAGAACGCCCCTACC -3’) and reverse (5’-AGGGGTTGGTGTAAAATAGG-3’), which was previously described by Motazedian et al. (13). Kinetoplast DNA was chosen as the molecular target, a 615 bp fragment for L. major and 744 bp fragment for L. tropica was amplified. The amplification conditions were 95°C for 5 minutes, followed by 40 cycles of denaturation at 94°C for 30 seconds, annealing at 60°C for 45 seconds, and extension at 72°C for 1 minute, with a final extension step at 72°C for 5 minutes. Two standard samples of parasites (L. tropica and L. major) and a negative control sample were used to monitor the reaction. Negative control contained primes, dNTP, MgCl2, Taq DNA polymerase, and distilled water. PCR products, by 100 bp DNA Ladder (Fermentas), were visualized by EthBr staining following 2% agarose gel electrophoresis. The results were analyzed by SPSS 16.
3.7. Ethical Approval
The experimental design for this study was approved by the ethics committee of Mashhad University of Medical sciences (IR.MUMS.REC.1392.805)
4. Results
4.1. Sampling and Parasitological Examination
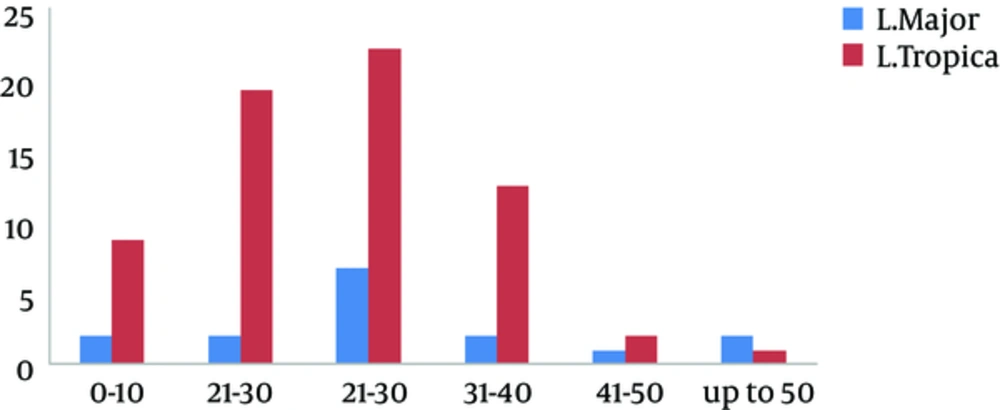
Of the 93 samples obtained from direct smears of suspected patients, 81 were positive in parasitological examination while 84 (68 cases (81%) L. tropica and 16 cases (19%) L. major) were positive in PCR directed to kDNA as shown in Table 1. Among the rest of the patients that were negative for leishmaniasis (9 cases), 2 of them had the Mycobacterium marinum infection on hands by culture on Lowenstein-Jensen medium. They are working in ornamental fish market. Among 84 individuals who had cutaneous leishmaniasis, 48 (57%) were male and 36 (43%) were female, where 37 (44%) came from rural areas and 47 (56%) were from urban regions. Most of the lesions were located on the hands (37%), face (29%), and feet (25%). The most clinical features of skin lesions were papule (75%) (P = 0.001) and ulcerated plaque (19%). Most of the people with CL were 21 - 30 years, as shown in Figure 2.
| Method | Positive | Negative | Total |
|---|---|---|---|
| Giemsa stain | 81 (87) | 12 (13) | 93 (100) |
| kDNA-PCR | 84 (90) | 9 (10) | 93 (100) |
Results of the Diagnostic Methods for Cutaneous Leishmaniasis in Patients with Suspected Lesion(s) in Gonabad, Kashmar and Bardaskan During 2015a
4.2. Molecular Diagnosis
PCR method was performed for diagnosis and characterization of Leishmania species on extracted DNA obtained from suspected lesions. The results showed that 68 isolates, 68 patients (81%) were L. tropica and 16 patients (19%) were L. major. 12 patients were from Bardaskan (10 cases had L.tropica), 23 patients from Gonabad (20 cases had L.tropica), and 49 patients from Kashmar (38 cases had L. tropica). Kashmar was a focus of ZCL with 22.5% L. major (Table 2).
| Cities | Number of Patients | L. tropica Agent | L. major Agent |
|---|---|---|---|
| Bardaskan | 12 | 10 | 2 |
| Gonabad | 23 | 20 | 3 |
| Kashmar | 49 | 38 | 11 |
| Total | 84 | 68 | 16 |
Results kDNA-PCR for Cutaneous Leishmaniasis in Gonabad, Kashmar and Bardaskan During 2015
5. Discussion
Characterization of Leishmania species and insect vectors is very helpful, due to the fact that different species have distinct treatment regimens and different prevention ways (6, 14). Intralesional injection of Glucantime was done more frequency for L. tropica (because of more drug resistance) rather than L. major. Furthermore, antibiotic therapy was proposed for treatment of superimposed bacterial wound in L. major lesions. It was reported that 4,900 CL cases were only from Mashhad (capital city of Khorasan province) and between 2000- 2002 with the most form of ACL (15). In another study from 21 positive samples, only 2 (9.5%) isolates were L. major in Mashhad (1) and from the 136 smears by the PCR method all of the positive samples were L. tropica (10). However, the present study showed a prevalence of 19% for L. major in Gonabad, Bardaskan, and Kashmar cities in southeast Khorasan.
Some surrounding cities of Mashhad have been reported as ZCL focus such as Dargaz, Sabzevar, Sarakhs, and Turkemen sahara (5, 12, 16). There was no information and exact data regarding etiological agents of leishmaniasis in above-mentioned cities. Already most patients who engaged with CL in these areas were ACL. Perhaps, Gonabad, Bardaskan, and Kashmar are small cities, they have close contacts between animal (Reservoir hosts of L. major), human, and Phlebotomus spp (vector host) rather than Mashhad city (the capital of Khorasan province). Therefore this study introduced a new foci of ZCL in the south and west of Khorasan. It was interesting to study the molecular diagnosis of Leishmania spp in rodents of this area. Almost all the cases of cutaneous leishmaniasis (90%) occur in only 7 countries such as Iran, Afghanistan, and Saudi Arabia (17).
Khorasan province is the neighbor of Afghanistan, and every year, many Afghan refugees come to these cities for work. Apparently, such challenges increase the spread and focuses of the disease. In our study, most of the patients were male (57%), similarly to other studies done in Isfahan, Shiraz, Damghan and Qom province in Iran (18, 19). The south and west of Khorasan has dry and warm weather, temperature above 40°C in the summer and near the great desert. Due to these conditions men who work in deserts and wastelands and wear fewer parts of clothing probably have more contact with sand flies during the evening rather than women. In the present study, most of the lesions were located on hands (37%); other studies in various parts of Iran have also suggested that the most of lesions of ACL occur in hands (20, 21).
kDNA- PCR sensitivities was described between 75% - 98% for detection of Leishmania species in many studies (22-25). Even kDNA was detected in the urine of patients with cutaneous leishmaniasis (26) and with visceral leishmaniasis (27). Recently, for the first time, real-time PCR was used for diagnosis and identification of Leishmania spp with a sensitivity of 98% (96/102) on Giemsa-stained slides that were stored for more than 3 years (28).
In this study, the results of kDNA-PCR approved that both diseases (ACL and ZCL) exist in the cities of Gonabad, Kashmar, and Bardeskan. Moreover, it seems that Mashhad is the important foci of ACL, while ZCL is more prevalent in the cities around Mashhad. Surrounding cities of Mashhad could be an important reservoir of ZCL for the Khorasan Province as a pilgrimage center for Muslims.
5.1. Conclusion
These data indicate that both L. tropica and L. major are the causative agents of cutaneous leishmaniasis in the Khorasan province. L. tropica is the dominant Leishmania species in Mashhad but in smaller cities of the Khorasan province ZCL focuses exists. Travelers in endemic areas should be advised that the only preventive measures are those of protection against sandflies bite. Leishmaniasis should be considered in travelers with compatible clinical findings and a history of travel to an endemic area, even months or years ago. Information regarding leishmaniasis and appropriate protective measures should be offered to adventure travelers, military personnel, and immigrants likely to be exposed to sandflies in endemic areas.