1. Background
Pertussis is one of the main reasons for infectious disease-related deaths in children (1). Bordetella causes this respiratory disease. Bordetella pertussis is a small Gram-negative pathogen (2, 3). The common signs of pertussis are paroxysmal cough, wheezing, and post-cough vomiting. Such clinical signs are not only limited to pertussis infection, but other pathogens, including the influenza virus, parainfluenza virus type III (PIV-III), respiratory syncytial virus (RSV), adenovirus (ADV), Mycoplasma pneumoniae, Chlamydophila pneumonia, and B. parapertussis can also cause similar clinical signs, which are collectively referred to as the pertussis-like syndrome (4, 5). The pertussis-like syndrome might happen at any age, but its prevalence is higher in children. It is challenging to differentiate the symptoms of B. pertussis from infections caused by viruses and some bacteria. Therefore, the pertussis diagnosis should not be solely based on the clinical signs, and the clinical diagnosis must be confirmed by laboratory tests (3, 5).
There is limited information on the etiology of pertussis-like syndrome worldwide (6). Furthermore, in Iran, culture and molecular methods are not routinely used in hospitals to confirm pertussis clinically, and they are only performed at the Pasteur Institute of Iran as a reference laboratory for pertussis. Therefore, accurate tracking of the prevalence of pertussis is difficult. In addition, it is important to know the pathogen causing the pertussis-like syndrome to reduce the rate of transmission and accelerate the start of treatment. Besides, any decision to change national vaccination plans, including the replacement of new vaccines, should be made based on the results of local epidemiological studies in each region.
2. Objectives
This study aimed to evaluate the frequency of pertussis-like pathogens among children admitted to a children's hospital in southwestern Iran.
3. Methods
This cross-sectional study aimed to identify the prevalence of the etiologic causes of the pertussis-like syndrome in children under the age of 5 years suspected of pertussis and admitted to Aboozar Children's Hospital between July 2018 and July 2019. This hospital is the only children's referral hospital in the Khuzestan province and is located in the city of Ahvaz. The inclusion criteria were coughs lasting more than 14 days with one or more of the following symptoms: Whoop and paroxysmal cough, apneic paroxysm, post-tussive vomiting, or coughs for more than 21 days without the symptoms mentioned above. Demographic, clinical, and laboratory data were collected from questionnaires filled out by the patients' parents and the medical records.
3.1. Sample Collection
Two nasopharyngeal samples were taken from the patients using a Dacron swab, and 2 samples were taken from each patient. One of the swabs was then placed in the Regan-Lowe transport medium to detect bacterial agents. After that, the second swab was placed in the viral transport medium (VTM) to detect viral agents. All the specimens were transferred to the laboratory.
3.2. Molecular Diagnosis
3.2.1. Polymerase Chain Reaction for the Identification of Bacteria
According to the manufacturer's instructions, deoxyribonucleic acid (DNA) was extracted using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). The DNA obtained was immediately assessed or stored at -80°C until use. Polymerase chain reaction (PCR) was performed with 5 μL of the template DNA. The used primers and conditions for B. pertussis and B. parapertussis were described by Castillo et al. and Dragsted et al. (7, 8). The A191-bp fragment of the pertussis toxin S1 gene (PTxA) and a 145-bp fragment of the insertion sequence IS481 were amplified using the primers. Samples were positive for B. pertussis when both the fragment of the pertussis toxin S1 gene (PTxA) and the insertion sequence IS481 were amplified. Primers BPPA amplify a 493 bp fragment of the insertion sequence IS1001, found in B. parapertussis (7-9). Moreover, the used primers and conditions for M. pneumonia and C. pneumoniae were described by Del Valle-Mendoza et al. (10).
3.2.2. Real-time PCR for the Detection of Adenovirus, Respiratory Syncytial Virus, and Parainfluenza Type III
Ribonucleic acid (RNA) extraction was performed using the High Pure RNA Isolation Kit (Roche Applied Science, Mannheim, Germany) based on the manufacturer's instructions. Next, RSV, PIV-III, and ADV were detected in the nasopharyngeal swab specimens using the real-time PCR described earlier (11). The primers and the probe for RSV and ADV were previously described by Liu et al. (12), and Heim et al. (13). For the case of PIV-III, the primers and conditions used for the real-time PCR were described by Coiras et al. (14).
3.3. Statistical Analysis
The data were analyzed in SPSS v. 22 (SPSS Inc., Chicago, IL, USA) and then expressed as appropriate numbers with percentages or medians and the interquartile range (IQR). Moreover, non-normally distributed variables and categorical data were analyzed using the chi-square test. A P-value < 0.05 was considered statistically significant.
4. Results
4.1. Demographic Characteristics
In this study, 45 eligible patients under the age of 5 years were examined. Of the 45 nasopharyngeal aspirates, 15 (33.3%) were B. pertussis-positive, referred to as the B. pertussis group; moreover, 30 (66.6%) children with other bacterial and viral pathogens or the absence of the studied microorganisms were classified into the pertussis-like group. Demographic data obtained from the study group are given in Table 1. The mean age of the children was 3.2 ± 2.5 months. Most children were in the age group of 1 to 8 months. The ratio of males to females was twice in the pertussis-like group; however, no significant differences regarding sex were observed between pertussis and pertussis-like groups (P = 0.51). All the patients were hospitalized for 3 - 7 days (mean: 3 days). In 17 (37.8%) patients, a history of coughs (lasting for more than 2 weeks) was observed in a family member 1 month prior to starting the study.
| Parameters | Total Patients | Pertussis Group (n = 15) | Pertussis-Like Group (n = 30) | P-Value |
|---|---|---|---|---|
| Age (mo) | 3.2 ± 2.5 | 2.837 | 3.155 | 0.152 |
| Sex (M/F) | 28/17 | 8/7 | 20/10 | 0.51 |
| History of antibiotic use | 28 (62.2) | 8 (53.3) | 20 (66.7) | 0.517 |
| Similar cases in the family | 17 (37.8) | 4 (26.7) | 13 (43.3) | 0.341 |
| Length of hospital stay | 5 (3 - 7) | 4.8 (3 - 7) | 5 (3 - 7) | 0.082 |
| Clinical symptoms | ||||
| Whoops | 2 (4.4) | 2 (13.3) | 0 | 0.106 |
| Paroxysmal cough | 8 (17.8) | 7 (46.6) | 1 (3.3) | 0.001 |
| Vomiting | 29 (64.4) | 12 (80) | 17 (56.6) | 0.189 |
| Apnea | 15 (33.3) | 4 (26.6) | 11 (36.6) | 0.738 |
| Cyanosis | 38 (84.4) | 15 (100) | 23 (76.6) | 0.077 |
| Fever | 16 (35.6) | 4 (26.6) | 12 (40) | 0.514 |
| Tachypnea | 15 (33.3) | 2 (13.3) | 13 (43.3) | 0.053 |
| Clinical signs | ||||
| Wheezing | 19 (42.2) | 2 (13.3) | 17 (56.7) | 0.005 |
| Crackles | 3 (6.7) | 0 | 3 (10) | 0.999 |
| Rhonchi | 2 (4.4) | 1 (6.7) | 1 (3.3) | 0.846 |
| Shaggy hearth | 5 (11.1) | 5 (33.3) | 0 | 0.026 |
| Parenchymal involvement | 2 (4.4) | 0 | 2 (6.7) | 0.7 |
| Laboratory values | ||||
| White blood cells median (interquartile range) | 11558 (5000 - 25300) | 11559 (5000 - 21000) | 11558 (5000 - 25300) | 0.782 |
| Lymphocyte median (interquartile range) | 64.5% (48 - 80) | 64.7 (48 - 78) | 64.5% (48 - 80) | 0.845 |
Demographic and Clinical Presentations of the Pertussis-Like Syndrome a
4.2. Comparison of the Clinical and Laboratory Characteristics Between Pertussis and Pertussis-Like Groups
Table 1 shows the distribution of signs and symptoms in the pediatric pertussis group and pertussis-like groups. Cyanosis (84.4%) and vomiting (64.4%) were the most common symptoms, and paroxysmal cough (17.8%) and whoop (4.4%) were the least common ones, respectively. Fever was present in only 16 cases (35.6%), and tachypnea was positive in 15 (33.3%) cases. There was no significant difference in terms of the clinical manifestations, including whoop, vomiting, apnea, cyanosis, fever, and tachypnea, between the two groups. Still, regarding paroxysmal cough, a significant difference was observed between pertussis and pertussis-like groups (P = 0.001). Lung hearing was normal in about half of the participants. Wheezing (42.2%) was the most common abnormal hearing problem, followed by crackling (6.7%) and rhonchi (4.4%), respectively. Among the symptoms mentioned above, there was a significant difference between the two Bordetella and Bordetella-like groups only in wheezing (P = 0.005). Chest X-ray (CXR) findings also showed that most cases were normal.
Only 15.5% of these cases showed some abnormal findings, including shaggy hearth and parenchymal involvement. Among those who were positive for B. pertussis, 10 cases had a normal radiograph, and 5 cases (33.3%) had a shaggy heart. About the shaggy heart, a significant difference was observed between these two groups (P = 0.026). Moreover, 40% of all the patients had a white blood cell (WBC) count below 10,000/mL; 49% of them had 15,000 - 100,000 WBC/mL, and 11.1 % had more than 150,000 WBC/mL. Among those with pertussis, only 3 children (20%) had more than 15,000 WC. No significant relationship was found between lymphocyte and leukocyte counts and the identification of B. pertussis as the causative agent of the disease (P = 0.78, P = 0.84).
4.3. Pathogens in the Children with the Pertussis-Like Syndrome
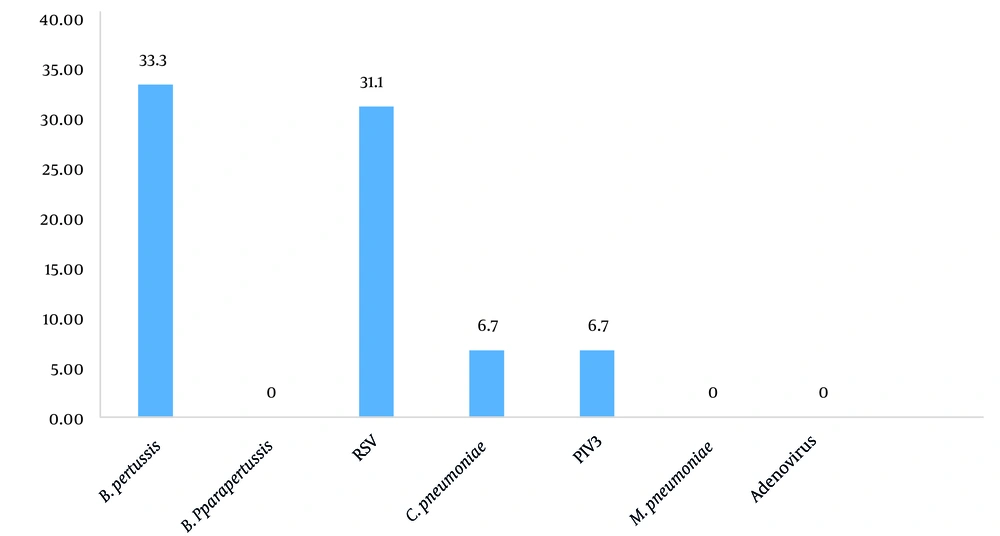
Of the 45 children with the pertussis-like syndrome, 35 (77.7%) children were positive for the pathogen. Bordetella pertussis (33.3%), RSV (31.1%), C. pneumonia (6.7%), and PIV-III (6.7%) were the microbial pathogens found. Moreover, 30 children were negative for B. pertussis, and all the children were negative for B. parapertussis. Adenovirus and M. pneumoniae were found in none of the subjects. Additionally, no co-infection was observed among the patients (Figure 1).
5. Discussion
This study aimed to investigate the microorganisms involved in the pertussis-like syndrome and evaluate the clinical characteristics in children under 5 years old in southwestern Iran. Our findings showed that B. pertussis (33.3%) and RSV (31.1%) were the most common microorganisms, and C. pneumoniae (6.7%) and PIV-III (6.7%) were the least common ones involved in the pertussis-like syndrome, respectively. The prevalence rate of B. pertussis in children with the pertussis-like syndrome was estimated as 33.3%, which is higher than those reported in similar studies by Mahmoudi et al. in Iran (18%), Hajia et al. in Iran (9%) and Al Maani et al. in Oman (17%) (3, 15, 16). However, in the current study, the estimated frequency was lower than those obtained by Gu et al. (53%) and Jiang et al. (49%) in China, Saiki-Macedo et al. in Peru (41%), and Dumaidi and Al-Jawabreh in Palestine (49%) (5, 17-19).
The differences in the prevalence of B. pertussis in the suspected patients may be due to the different age ranges of the studied populations, the use of various diagnostic methods with different percentages of sensitivity and specificity, and differences in vaccination coverage in the studied regions. In addition, the transmission of pertussis from adults to infants may increase due to the hot climate in Ahvaz, the presence of more people in their houses, and, consequently, insufficient indoor air ventilation. In Iran, the pertussis vaccination program includes a total of 3 doses of the diphtheria and tetanus vaccine, combined with whole-cell pertussis vaccine, which is injected at 2, 4, and 6 months. In addition, booster doses are given at 18 months and 4 - 6 years old (20).
In the present study, 86% of the cases with confirmed pertussis-like syndrome (by PCR) were 0 to 4 months old and had not received the full vaccination dose. Due to the reduced efficacy of the pertussis vaccine with aging, adults play an important part in transmitting this disease to infants, especially those who have not received full vaccination (21). Therefore, some strategies should be used to support infants, including the vaccination of adolescents and adults with the acellular pertussis vaccine to prevent the home transmission of the disease, as well as the vaccination of pregnant women during the last trimester of their pregnancy; the latter measure results in the transmission of maternal anti-pertussis antibodies to the fetus and, as a result, the newborns are protected against pertussis before being vaccinated (20, 22).
Since the whole-cell pertussis vaccine is not recommended for adults and there is no access to an acellular vaccine in Iran, it is necessary to start the vaccination program in the target groups with this type of vaccine and include it in the national vaccination program. In this study, RSV accounted for a high percentage of positive cases, as it was identified in 31% of cases. Similar results have been reported in Brazil (32%), China (31.4%), and Oman (32%) (16, 23, 24). The studies conducted by Mahmoudi et al. (20%) and Pourakbari et al. (17.2%) in Iran showed a lower rate. However, in these two articles, RSV was the most common viral pathogen (3, 25). In our study, PIV-III was detected in 6.7% of the children with the pertussis-like syndrome; this rate is lower than that of the parainfluenza virus obtained in Tao et al.'s (24) study in China (43%) and higher than that of Gu et al.'s study in China (3.2%) (5, 24).
Chlamydophila pneumoniae was also identified in 6.7% of the children with the pertussis-like syndrome. A similar study in Peru identified 10.5% of this bacterium among children (10). In the present study, M. pneumoniae and adenovirus were detected in none of the subjects. Nevertheless, in similar studies, Mycoplasma was reported in 29.2% of the children with pertussis-like syndrome in China and 26% in Peru (17, 18); adenovirus was also identified in 49% of subjects in Peru, 3.2% in Oman, and 16% in Iran (3, 16, 18). As the etiology of pertussis, B. parapertussis is rare (26, 27). In our study, B. parapertussis was found in none of the participants.
However, paroxysmal cough and whoop are the important symptoms of pertussis and are used in the clinical definition of pertussis provided by the World Health Organization (WHO). In our study, only 13% of the patients with B. pertussis had whoop. Moreover, a significant difference was observed regarding paroxysmal cough between pertussis and pertussis-like groups, as about half of the patients with B. pertussis had this symptom. Regarding the other clinical symptoms, no significant difference was observed between the two study groups. This result is consistent with those of other studies in Iran and other parts of the world, showing a lack of specificity of clinical symptoms in pertussis diagnosis. This indicates the need for microbiological tests to confirm the diagnosis of suspected pertussis (3, 5, 22, 28, 29).
The present study observed no significant relationship between WBC, absolute lymphocyte counts, and B. pertussis positivity. However, some studies have shown that these two indexes can be used in laboratory separation of B. pertussis from other bacteria causing the pertussis-like syndrome (30). However, in 15 children with positive PCR test results for B. pertussis, the mean WBC count was 11,559. The mean lymphocyte percentage was 64.7%, which is higher than the average typical condition in pertussis (31). Accordingly, the lack of significance of these two parameters in the B. pertussis and pertussis-like groups can be explained by the fact that in this study, there was no healthy control group in which we assessed the percentages of WBC and lymphocytes. Therefore, a comparison of leukocytes and lymphocytes was made between the B. pertussis group and the pertussis-like group. The second group showed leukocytosis and lymphocytosis due to other bacteria and viruses, especially RSV. Chest X-ray changes were also found to be associated with Bordetella positivity. The classic Bordetella modification is a shaggy heart. Furthermore, diffuse infiltration and flattening of the diaphragm are known as the common causes of this bacterium. Still, its prevalence may not significantly differ from other causes of pertussis syndrome (30). In our study, there was a significant difference between the two study groups in terms of the shaggy heart.
A limitation of the present study was its small sample size. In the next stage, we should include more children suspected of having pertussis-like syndrome. In addition, we did not examine all the pathogens involved in the pertussis-like syndrome, so we should detect more kinds of pathogens involved in the pertussis-like syndrome later.
5.1. Conclusions
The findings of this observational and prospective study showed that B. pertussis and RSV, with almost similar prevalence rates, are the causes of approximately 64% of the cases of pertussis-like syndrome in children under 5 years old hospitalized in Ahvaz. This finding should be considered in the clinic. Furthermore, clinically suspected cases should be confirmed using molecular methods.