1. Background
The novel coronavirus disease 2019 (COVID-19) pandemic has spread worldwide. Currently, the best pre-exposure preventive strategies for this global threat are vaccinating and implementing hygienic recommendations, such as using face masks, keeping physical distance, rapid identification of cases, and isolation (1, 2). The post-exposure prophylaxis for COVID-19 is essential and challenging. Several agents, including hydroxychloroquine, have been investigated, but none have been shown to prevent or mitigate post-exposure COVID-19 infection (3, 4). Although monoclonal antibodies were first recommended to be used as post-exposure prophylaxis (PEP) against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the United States National Institute of Health (NIH) panel now recommends against the use of these agents (5, 6).
The virus binds to human angiotensin-converting enzyme 2 (ACE2) receptors via its spike glycoprotein (S-Protein). After binding, the virus can enter the cell via two different pathways. In the endosomal pathway, virus entry is mediated by the activation of cathepsin (cysteine protease), which causes membrane fusion and endocytosis. In the non-endosomal pathway, transmembrane protease serine 2 (TMPRSS2) facilitates priming the spikes, and virus entry occurs through the plasma membrane. Newer viruses tend to infect the cell through serine protease activation. Previous studies showed that TMPRSS2 inhibitors could block SARS-CoV-2 from cell entry (7, 8).
It has been observed that bromhexine can prevent influenza infection (9). Therefore, bromhexine has been proposed to prevent or treat COVID-19 infection. Clinical studies' results on bromhexine efficacy in hospitalized symptomatic patients with COVID-19 are conflicting (10-13). The other significant concern is the emergence of variants of SARS-CoV-2, such as Delta and Omicron. Any mutation in the spike protein area may harm the efficacy of vaccines and monoclonal antibody treatments against the new variants (14).
2. Objectives
Blocking non-endosomal cell entry of the virus might be an effective method to counter new virus variants. We hypothesized that bromhexine could be utilized as an affordable, widely available prophylactic agent to prevent symptomatic infection after exposure to SARS-CoV-2. Hence, we aimed to assess the efficacy of this agent in a randomized controlled clinical trial.
3. Methods
3.1. Study Design
A multi-center randomized, double-blind, placebo-controlled clinical trial was conducted to evaluate the efficacy of bromhexine for post-exposure prophylaxis against COVID-19. The study's methodological framework was modified and partially adopted with permission from the Boulware et al. (3) study, which was published in the New England Journal of Medicine [NEJM] on June 3, 2020. All participants were randomly assigned in a 1:1 ratio to receive either bromhexine or placebo. All participants had exposure to a household member confirmed with COVID-19 by polymerase chain reaction (PCR) assay.
3.2. Patient Selection and Setting
In this trial, asymptomatic individuals ≥ 18 years old who had close contact with a hospitalized household member with confirmed COVID-19 within four days were recruited and followed up. Close contact was defined as exposure at fewer than 2 m for more than 15 minutes in 24 hours. All participants agreed not to enroll in another study of an investigational agent before the completion of day 14 of the study. The exclusion criteria were an age of below 18 years, current hospitalization, experiencing signs or symptoms of COVID-19 within 30 days prior to and on the day of screening, receiving any experimental treatment for COVID-19 (off-label, compassionate use, or trial-related) within 30 days prior to the screening, and history of allergy to cough syrup.
Participant recruitment was performed by tracking household members of patients with confirmed COVID-19 based on PCR results within four days of exposure. Written informed consent was obtained from the individuals before study recruitment. All participants were given their medication/placebo on enrollment day. Participants were subsequently followed in virtual visits on days 5, 10, and 14 for the signs and symptoms of COVID-19, medication adherence, COVID-19 testing, hospitalization, and adverse drug reactions. Participants had no routine screening for COVID-19 during the 14-day follow-up unless symptomatic enough to seek care.
3.3. Intervention
The random allocation sequence was generated using Sealed Envelope Ltd.'s online service. A permuted block randomization sequence with a variable block size containing four, six, or eight patients was used. All participants, investigators, outcome assessors, and data analyzers were blinded to the assignment. Only one person was aware of the randomization sequence. This individual was not aware of the patients' recruitment and follow-up process.
Bromhexine and an identical placebo were purchased from Mofid Nikan Pharmaceutical Company (Tehran, Iran). All medications were packaged and labeled identically (except for the active ingredient component), and the medication was not distinguishable from the placebo. The medication was in a sealed, masked bottle containing 50 bromhexine hydrochloride or placebo tablets. The pill count method was used to evaluate the adherence of the participants. All bottles had a distinctive label as a serine protease inhibitor and were dispensed directly to the participants on day 0 by the investigators. A bromhexine hydrochloride tab [8 mg] or a placebo tab was taken three times a day for two weeks.
3.4. Outcomes
The primary outcome of this study was defined as the incidence of symptomatic COVID-19 based on compatible signs and symptoms during the study period. The signs and symptoms of possible SARS-CoV-2 infection were considered as acute-onset cough and fever or two of the following symptoms: fever, chills, dyspnea, general weakness/fatigue, headache, myalgia, sore throat, and recent onset of anosmia or ageusia in the absence of any other possible identifiable cause. Reverse transcriptase-polymerase chain reaction (RT-PCR) assays were used to confirm the diagnosis of COVID-19.
Secondary outcomes included hospitalization or death, confirmed COVID-19 by PCR in symptomatic patients, all-cause medication discontinuation or withdrawal, adherence to the instructions of the medication use, and adverse drug reactions. Side effect data were collected by a direct question about adverse events, with open-ended free text.
3.5. Sample Size
Assuming that COVID-19 would develop in 10% of participants who have close exposure, a sample size of 435 individuals in each arm was calculated using Fisher's exact test with a 50% relative effect size to reduce new symptomatic infections, 80% power, and a 0.05 two-sided alpha. A 20% dropout rate increased the final enrollment size to 525 persons per arm.
3.6. Interim Analysis
With the spread of the highly contagious Delta variant of SARS-CoV-2, finding asymptomatic household contacts was a challenge in the setting of delta infection, and hence a request was made to the research and safety monitoring board to conduct an interim analysis. This unplanned interim analysis of the primary outcome (symptomatic COVID-19) with 187 and 185 participants in the bromhexine and placebo groups, respectively, was performed, and the trial was halted.
To account for the unplanned nature of the analysis, the significance level of 0.05 was adjusted to 0.03. Pocock published nominal significance levels required for repeated two-sided significance testing, which preserve an overall significance level (alpha) of 0.05 (15). These significance levels are stopping rules and can be used for binary or normally distributed response variables. When the maximum number of tests to be performed is two (and the desired overall alpha is 0.05), the nominal significance level for each repeated test is 0.029. This value was rounded to 0.03 and used as the significance level for the analyses in this paper.
3.7. Statistical Analysis
Statistical analysis was primarily performed using SPSS 25. Qualitative variables were analyzed by the chi-square or Fisher's exact test, as appropriate. Age as a continuous variable was presented as the median and interquartile range (IQR). The relative risk (RR) reduction and absolute risk reduction of developing a symptomatic COVID-19 infection were calculated. The number needed to treat (NNT) was computed as the reciprocal of the absolute risk reduction.
Logistic regression analyses were performed using SAS 9.4. The four binary outcomes in these models were a symptomatic case of COVID-19, a PCR-confirmed case of COVID-19, admission to a medical center, and death. Odds ratios (ORs) comparing patients in the bromhexine arm with those in the placebo arm were reported along with 97% confidence intervals (CIs) and p-values. Given the rarity of two outcomes under study (admission to a medical center and death), Firth's penalized maximum likelihood estimation was used when fitting these two models to avoid triggering sparse data bias (16). An OR was considered statistically significant if the p-value was less than or equal to 0.03.
Imbalances between the two study treatment arms in the prevalence of the following factors deemed to be of clinical importance: male sex, smoking, hypertension, and diabetes. These imbalances may have led to confounding. Assessment of confounding is not an issue of statistical significance, and therefore, the change-in-estimate method was used to determine if joint confounding by the four variables listed above was present (17). If the unadjusted and adjusted bromhexine ORs for a particular outcome differed by 10% or more, the variable or variables of adjustment remained in the logistic regression model, and the adjusted OR was reported. Per-protocol analyses were performed, given that subjects who consumed trial medications for less than three days were excluded from the study.
4. Results
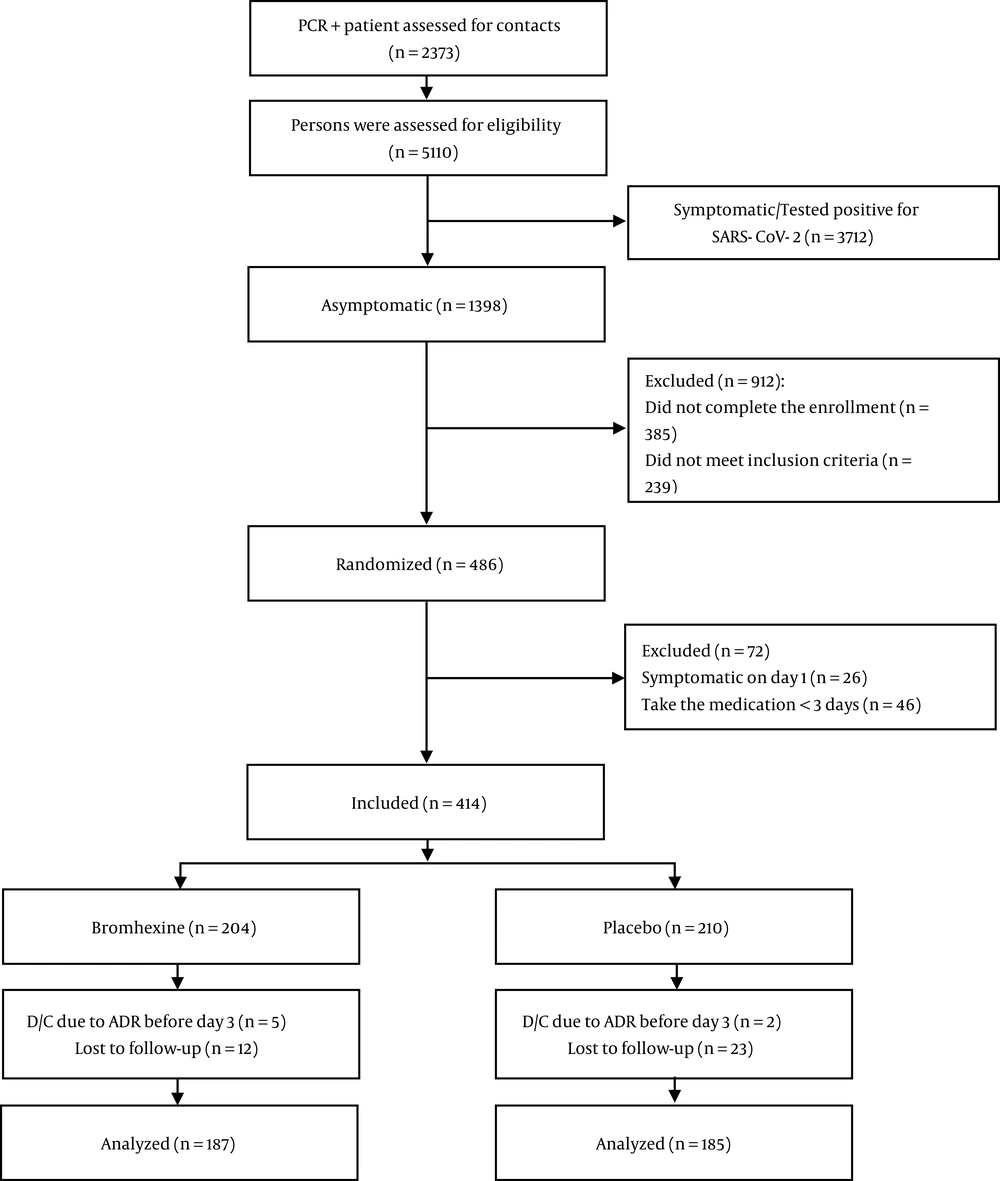
We evaluated 5,110 subjects who were in close contact with 2,373 PCR-positive COVID-19 patients. Eventually, 372 asymptomatic subjects were recruited and randomly assigned to bromhexine (n = 187) or placebo (n = 185). The study diagram is presented in Figure 1. The patients' median age (IQR) was 40 (21) years. Out of 372 participants, 198 (53.2%) were male, and 174 (46.8%) were female. The baseline demographics and clinical parameters of each group are reported in Table 1. Two hundred and forty-one (64.8%) subjects had moderate-risk exposure. Most participants were enrolled in the first two days after exposure to a COVID-19 patient (262, 70.4%).
| Characteristics | Bromhexine (n = 187) | Placebo (n = 185) |
|---|---|---|
| Age, y (IQR) [Min, Max] | 40 (21) [19, 82] | 41 (20) [18, 86] |
| Time from exposure variable (IQR) [min, max] | 1.0 (2) [0, 4] | 2.00 (2) [0, 4] |
| Body mass index (kg/m2) | ||
| < 30 | 162 (86.7) | 155 (83.8) |
| ≥ 30 | 25 (13.4) | 30 (16.2) |
| Sex | ||
| Female | 80 (42.8.0) | 94:91 (50.8) |
| Male | 107 (57.2) | 91 (49.2) |
| Habits | ||
| Current smoker | 26 (13.9) | 20 (10.8) |
| Opioid user | 6 (3.2) | 5 (2.7) |
| Alcohol | 3 (1.6) | 1 (0.5) |
| Past medical history | ||
| Hypertension | 19 (10.2) | 14 (7.6) |
| Diabetes mellitus | 14 (7.5) | 20 (10.8) |
| Coronary artery disease | 17 (9.1) | 14 (7.6) |
| Chronic kidney disease | 2 (1.1) | 4 (2.2) |
| Asthma | 2 (1.1) | 2 (1.1) |
| COPD | 2 (1.1) | 0 (0.0) |
| Immunocompromised | 0 (0.0) | 3 (1.6) |
| Malignancy | 1 (0.5) | 1 (0.5) |
| Past medication history | ||
| ARB/ACEi | 16 (8.6) | 14 (7.6) |
| Aspirin | 21 (11.2) | 18 (9.7) |
| Non-steroidal anti-inflammatory drugs | 6 (3.2) | 9 (4.9) |
| Other | 47 (25.1) | 40 (21.6) |
| Risk of exposure | ||
| High | 72 (38.5) | 59 (31.9) |
| Intermediate | 115 (61.5) | 126 (68.1) |
Abbreviation: IQR, interquartile range.
a Values are expressed as No. (%).
The overall incidence of being a symptomatic participant based on compatible signs/symptoms of COVID-19 was 13.4%. The incidence of COVID-19 was significantly lower in individuals who received bromhexine than in those who received the placebo (16 [8.6%] vs. 34 [18.4%], RR=0.47, P = 0.005). The relative risk reduction was 53%, while the relative risk reduction within five days of the initiation of bromhexine was 63%. The timing of the onset of COVID-19 (counts of new cases by trial day) during the study by treatment group is presented in Figure 2. PCR confirmation was reported in 13 (7.0%) and 26 (14.1%) of the symptomatic participants in the bromhexine and placebo groups, respectively (P = 0.025). The relative risk reduction for the outcome of being a PCR-confirmed case was 50%. The hospitalization rate did not vary significantly between the treatment arms [bromhexine: 1 (0.5%); placebo: 6 (3.2%), (P = 0.067)]. The incidence of death was 0 (0.0%) in the bromhexine group and 1 (0.5%) in the placebo group (P = 0.497). The decedent was a 53-year-old woman with diabetes who passed away due to COVID-19 complications after hospitalization and ICU admission.
Table 2 presents bromhexine vs. placebo ORs for four outcomes. The ORs for becoming a symptomatic case and PCR-confirmed case of COVID-19 are unadjusted, while the OR for admission to a medical center is adjusted for the patient's sex, diabetes, and smoking. The bromhexine OR for death is adjusted for the patient's sex, diabetes, and hypertension. The odds of becoming a symptomatic case of COVID-19 were reduced by 58% in participants who received bromhexine compared to those who received the placebo: OR = 0.42, 97% CI: 0.21 - 0.84, P = 0.007. A statistically significant reduction in the odds of becoming a symptomatic case of COVID-19 persisted after controlling for smoking, sex, hypertension, and diabetes: Adjusted bromhexine vs. placebo OR = 0.44, 97% CI: 0.22 - 0.88, P = 0.01.
| Outcome | Events in Bromhexine Arm | Events in Placebo Arm | OR | 97% Confidence Interval | P-Value |
|---|---|---|---|---|---|
| Symptomatic case of COVID-19 | 16 | 34 | 0.42† | 0.21 - 0.84 | 0.007 |
| PCR-confirmed case of COVID-19 | 13 | 26 | 0.46† | 0.21 - 0.99 | 0.028 |
| Admission to a medical center | 1 | 6 | 0.29* | 0.05 - 1.83 | 0.145 |
| Death | 0 | 1 | 0.67‡ | 0.04 - 11.03 | 0.759 |
a Absolute numbers of outcome events are presented by the study arm. †Unadjusted. *Adjusted for the patient's sex, diabetes, and smoking using logistic regression with Firth's penalized maximum likelihood estimation. ‡ Adjusted for the patient's sex, diabetes, and hypertension using logistic regression with Firth's penalized maximum likelihood estimation.
In the 50 symptomatic cases, fever 39 (78.0%), Chills 29 (58.0%), fatigue and weakness 27 (54.0%) were the most frequently reported symptoms. Participant self-report adherence is represented in Table 3. Furthermore, a post-hoc calculation for the primary outcome of symptomatic infection for an alpha of 0.03 (two-sided testing) revealed a power of approximately 73% (and for the same outcome in our study, the power, if one assumes an alpha of 0.05, would be about 79%).
| Variables | Bromhexine (n = 187) | Placebo (n = 185) | P-Value |
|---|---|---|---|
| Median duration of study participation in days (IQR)*[Range] | 14 (6) [3 - 14] | 10 (7) [3 - 14] | 0.007 |
| Adherence to trial intervention** | 140 (74.9) | 131 (70.8) | 0.379 |
| Average cumulative trial intervention adherence, % | 88.00 | 83.24 | <0.001 |
| Reasons for medication discontinuation | |||
| Adverse drug reactions | 5 (2.7) | 3 (1.6) | 0.724 |
| Felt no longer at risk | 38 (20.3) | 40 (21.6) | 0.758 |
| Advised not to take the medication | 7 (3.7) | 5 (2.7) | 0.570 |
| Adverse Drug Reactions | |||
| Any | 27 (14.4) | 19 (10.3) | 0.222 |
| Nausea/Vomiting | 6 (3.2) | 4 (2.2) | 0.751 |
| Upset stomach | 5 (2.7) | 3 (1.6) | 0.724 |
| Abdominal discomfort | 12 (6.4) | 8 (4.3) | 0.371 |
| Diarrhea | 8 (4.3) | 4 (2.2) | 0.248 |
| Constipation | 0 (0.0) | 1 (0.5) | 0.497 |
| Irritability | 1 (0.5) | 0 (0.0) | 1.000 |
| Paresthesia | 1 (0.5) | 0 (0.0) | 1.000 |
| Myalgia | 0 (0.0) | 1 (0.5) | 0.497 |
| Dizziness | 3 (1.6) | 2 (1.1) | 1.000 |
| Headache | 4 (2.1) | 1 (0.5) | 0.372 |
| Vertigo | 2.0 (1.1) | 0 (0.0) | 0.499 |
| Dry throat | 2 (1.1) | 1 (0.5) | 1.000 |
| Increased respiratory secretion | 2 (1.1.) | 0 (0.0) | 0.499 |
| Skin sensitivity/rash | 0 (0.0) | 1 (0.5) | 0.497 |
a Values are expressed as No. (%) unless otherwise indicated. *IQR, interquartile range; ** Participants who consumed ≥ 80% of assigned medication were considered adherent to the medication regimen.
4.1. Adverse Events
Adverse drug reactions occurred in 46 (12.4%) participants. Abdominal discomfort was the most frequently reported adverse reaction (Table 3).
5. Discussion
Our study evaluated the clinical efficacy of oral bromhexine hydrochloride in the post-exposure prophylaxis of COVID-19 infection. By blocking TMPRSS2, bromhexine inhibits virus entry into the cell and mitigates or prevents SARS-CoV-2 infection. In addition, bromhexine may be essential in restoring airway surface liquid and enhancing mucociliary transport by inhibiting the epithelial sodium channel (ENaC). These channels are upregulated and are activated by serine proteases (18, 19). It has been shown that the spike protein of SARS-CoV-2 at the cleavage site has the same sequence of amino acids as that found at its cut site in ENaC-α. It has been postulated that COVID-19, by hijacking TMPRSS2, activates ENaC and interferes with the water and salt balance in the lungs (20).
Our relative risk reduction within five days of bromhexine initiation was 63%. Furthermore, the incidence of symptomatic COVID-19 in our study was 8.6% in the bromhexine group and 18.4% in the placebo group (absolute risk reduction = 9.8%). The NNT in our study (1/0.098) was 10.2, indicating that, on average, 10 patients must be treated with bromhexine to prevent one case of symptomatic disease. The higher risk of symptomatic illness in both treatment and placebo arms in our study compared to the previously performed studies on other medications used for PEP might be explained by the spread of the highly contagious delta variant (5).
The safety profile of the medication which is used for new indications is an important area of concern. Bromhexine hydrochloride has been used since 1963 with an excellent safety profile (21), while the newer medications' administration could be accompanied by serious adverse drug reactions.
One small single-center, open-label, randomized clinical trial observed a lower intensity of lung involvement, such as cough, dyspnea, and lassitude, in hospitalized patients with COVID-19 who received bromhexine (11). In our trial, bromhexine protected against the development of the primary outcome of the symptomatic illness, OR=0.42. Taking the reciprocal of this OR (1/0.42) reveals that patients in the placebo arm had more than twice the odds of becoming a symptomatic case of COVID-19 than patients in the bromhexine arm (OR = 2.38). This protective effect of bromhexine on the primary outcome persisted even after controlling for smoking, sex, hypertension, and diabetes (aOR = 0.44).
Our data analysis also showed that the number of positive PCR results (confirmed cases) was significantly lower in participants who received bromhexine treatment than in the placebo group. This finding may reflect the mitigation or a way to break the chain of virus transmission in the participants who received bromhexine. In a recently published study, Mikhaylov et al. reported a lower frequency of positive PCR tests and symptomatic SARS-CoV-2 infection among medical personnel (22). In the study, bromhexine hydrochloride was administered at the dose of 8 mg three times a day. The primary endpoint of this study was a positive nasopharyngeal swab PCR test for SARS-CoV-2 or signs of clinical infection within 28 days and at week 8. They concluded that bromhexine administration is associated with a significant reduction in the rate of symptomatic COVID-19. Considering the small sample size as the main limitation of the study, which was underpowered, the difference between the two groups for positive PCR results was not statistically significant.
In addition to our data collection on self-report by all participants, the lack of serologic tests to assess the immunity of the individuals against COVID-19 prior to enrollment is one of the limitations of our study. To minimize those issues, any individuals with any signs or symptoms suggestive of COVID-19 infection within one month prior to the enrollment date were not included. Also, the random allocation of the participants into the two study groups reduced the chance of confounding bias by immunity/immune status. Similarly, it is to be expected that the prevalence of non-COVID-19 diseases, which could mimic the signs and symptoms of COVID-19, such as influenza and the common cold, would be distributed equally between the two groups. Also, we should consider that, based on national health organization reports, the prevalence of these two diseases in the pandemic of COVID-19 was low.
There is no doubt that the administration of vaccines against COVID-19 is the most effective, valuable, and cornerstone to preventing COVID-19 (23) and saving lives. However, the new SARS-CoV-2 variants, such as Omicron, with their higher transmissibility rate, might negatively affect the efficacy of vaccines and monoclonal antibodies (24). Several clinical trials are being performed and underway to test new antiviral medications for post-exposure treatment and treatment of patients with risk factors for severe disease (25). Therefore, it is judicious to have another layer of post-exposure protection, a medication with a safety profile that has been tested for more than half a century.
5.1. Conclusions
Our data demonstrated the benefits of using bromhexine as post-exposure prophylaxis. Access to available and inexpensive oral medications such as bromhexine may provide another effective layer of protection in ending the pandemic especially given the emergence of new variants and the mass vaccination challenges in developing countries. Further large-scale randomized controlled trials using definitive laboratory confirmation and meta-analysis might be needed.
![Symptomatic COVID-19 infection in the bromhexine and placebo groups. The cumulative number of symptomatic COVID-19 cases [50 patients] over two weeks of follow-up in both bromhexine and placebo groups. Symptomatic COVID-19 infection in the bromhexine and placebo groups. The cumulative number of symptomatic COVID-19 cases [50 patients] over two weeks of follow-up in both bromhexine and placebo groups.](https://services.brieflands.com/cdn/serve/3170b/90e9e43f0310ca64a8d6b30aea49df9c48e9e58b/jjm-130198-i002-F2-preview.webp)