1. Background
Herpes simplex virus (HSV) is a member of the human herpesvirus family. HSV is an enveloped, spherical virion with complete 152-kbp genomic DNA (1). During the initial infection of new host cells, HSV increases rapidly in the cell nucleus. The HSV genome is composed of 3 stages: immediate-early (IE), early (E), and late (L) genes. The virus frequently remains latent in the neurons until it restimulates a replication cycle (2). As a world health problem, HSV infection is detected in about 50% to 90% of people who are serum positive for HSV-1 (3, 4). The infection emerges with different degrees of clinical features, from mild to severe, including herpetic gingivostomatitis, herpetic keratoconjunctivitis, and herpes encephalitis. Therefore, the use of novel antiviral drugs as alternative treatments for successful therapy is needed to minimize the development of resistant acyclovir (ACV) in these patients and severe infections in immunocompromised transplant recipients or HIV-infected individuals (5).
Synthetic nucleoside analogues (such as ACV, valacyclovir, and famciclovir) are available. They are used as an anti-herpes compound to treat various HSV infections, but extensive clinical use of this compound can develop drug-resistant viruses (6, 7). The percentage of HSV resistance to ACV among general immunocompetent patients is usually low. However, the resistance ratio has been dramatically increasing and has significantly increased from 5% to 14% in bone marrow transplant recipients (BMT) receive (8, 9). The emergence of ACV-resistant strains has led to long-term prophylaxis and treatment with ACV-expected relapses and failure in treatment procedures (10). Hence, the transfer of ACV drug-resistant strains to susceptible people should also be considered.
According to the literature, there is a need for a new and effective antiviral agent to either replace or complement conventional antiherpes drugs (11, 12). It can be suggested that alternative antiviral medications can help successful therapy and minimize the appearance of resistant ACV in patients. Natural products are the main sources of new components purified and identified as active components from raw natural materials such as plant parts or divided marine sources (13-15). These ingredients are a useful source of medical treatment, and several of today’s medicines and drugs are natural product derivatives. Therefore, there is a need for new and advanced antiviral effects, antiviral drug targets, and antiviral mechanisms (9, 16). A molecule called (S)-10-hydroxycamptothecin (10-HCPT), camptothecin (CPT), specifically blocks topoisomerase I that breaks and leaves the DNA strand during the replication stage. A few studies have reported that CPT inhibits the replication and packaging of double-stranded and single-stranded DNA containing HSV-2, adenovirus viruses, papovaviruses, and autonomous parvovirus.
2. Objectives
This study examined the potential anti-herpesvirus activity of natural products in an extensive library of 133 compounds by examining viral titers and the number of viral plaques. It also provided insight into the mechanism of action of the most potent compounds on HSV-1 and demonstrated an inhibitory viral replication step.
3. Methods
3.1. Cells
A549 and Vero cells were obtained from the Pasteur Institute of Iran, and primary rabbit kidney (PRK) cells were gifted by the Center for Comparative Experimental Medicine, Iran. The cells were cultured in 1% penicillin-streptomycin (Penstrep; 10 000 U/mL, Gibco) supplemented with DMEM (Gibco, Grand Island, NY) and bovine serum albumin (BSA, Gibco) at 37°C and in 5% CO2. PRK cells were derived from the kidneys of a 1-week-old baby rabbit anesthetized by ketamine hydrochloride (5 mg) as an intramuscular injection of 10-mg xylazine. Each kidney was removed, chopped, and placed in a trypsin buffer. Tissues were isolated by incubating at 37°C on an Esterer device. The cells were centrifuged (1500 rpm) for 20 minutes at 25°C and then collected. The PRK cell aliquot was then stored at 80°C until further use. Finally, the PRK cells were cultured in 1% Penstrep (10 000 U/mL, Gibco) supplemented with DMEM (Gibco, Grand Island, NY) and supplemented with 15% fetal bovine serum (Gibco) at 37°C in 5% CO2.
3.2. Virus Strain
The HSV used in this study was isolated from the patient who was previously characterized (17) and diagnosed. The virus was purified with type-specific primers and probes in a real-time polymerase chain reaction (PCR)-based system (Applied Biosystem, CA, USA) (18). Next, direct immunofluorescence staining was performed using type-specific monoclonal antibody agents HSV-1/HSV-2 (catalog number: K6106112, Thermo Scientific). The ACV susceptibility was then assessed by a plaque reduction assay (19). Genotyping was performed on thymidine kinase and DNA polymerase genes from HSV. Prototype HSV-1 was named as an HSV-1AN95 sensitive laboratory strain. The virus was then propagated in Vero cells and titrated according to the endpoint dilution (20). The cells were then aliquoted at 70°C for later use. Overall, the ACV (0.01 mg/mL) assay was considered a gold standard treatment to inhibit viral growth.
3.3. Primary Screening for Antiviral Activities
Cytopathogenic effect (CPE) reduction as a read-out was used to screen a total of 133 natural products (Selleckchem Natural Product Library, Catalog No. L1400) to find out the inhibitors of HSV-1. Briefly, 5 × 105 A549 cells were seeded into a 96-well plate (SPL Life Sciences) and incubated at 37°C with 5% CO2 24 hours before inoculation. Two concentrations (5 µM and 30 µM) of each compound (solved in assay media with a final dimethyl sulfoxide (DMSO) concentration of 0.1% and 0.018%, respectively) were introduced into the plates (1 compound for each well). Before virus inoculation, each test was well treated with a natural product and incubated at 37°C for 2 hours.
The cells adapted to the new media with the natural product. Then, 10 µL of the diluted virus with 0.5 multiplicity of infection (MOI) was added to the wells. The DMSO control consisting of DMSO, virus, and cells were set up in each plate for all the performed assays. The final assay volume was 200 µL/well. Then, 0.05% and 0.009% DMSO were added to the cell control wells and DMSO wells, respectively. After incubating at 37°C for 48 hours; then, the plates were saved at -70°C with 3 freezing and thawing stages to isolate the HSV rapidly. The assay was performed by measuring the progeny viral titer using the endpoint dilution method (20). Vero cells (5 × 105) were placed into a 96-well plate (SPL Life Sciences). The cells were incubated at 37°C with 5% CO2 for 24 hours. The serial dilutions of each well on A549 cells were obtained in 96-well plates separately. Then, the 96-well plates were incubated at 37°C for 48 hours; afterward, each well was fixed with 300 mL of formaldehyde solution (10%) and stained with crystal violet (0.5%) in 20% methanol. These plates were monitored, and compounds with > 80% inhibition compared to DMSO were picked as initial hits.
3.3.1. Cytotoxicity Assay
The A549 and primary cells were used to determine the cytotoxicity of 10-HCPT in vitro by a WST-1 test (Roche Applied Science, Canada). Briefly, 5 × 103 A549 and PRK cells were added to a 96-well plate. Afterward, they were incubated with various concentrations of 10-HCPT at 37°C. After 24 hours, 10 µL of WST-1 reagent was poured into the wells and incubated for 4 hours. Then, the cells were entirely shaken in a shaker for 1 minute, and the absorbance was read at 440/640 nm (BioTek, Epoch Microplate Spectrophotometer Fisher Scientific). The 50% cytotoxic concentration (CC50) of 10-HCPT was calculated on the A549 and PRK cells.
3.3.2. Virus Yield Reduction Assay
This assay was performed as previously described (21). Briefly, a 70% confluent A549 cell monolayer was prepared in a 24-well plate prior to virus inoculation. Each test well was treated with different concentrations of 10-HCPT (0.01 μM, 0.03 μM, 0.06 μM, 0.12 μM, 0.24 μM, and 0.48 μM) and incubated at 37°C for 2 hours to adapt to the new media. Each well was filled with 25 µL of the diluted virus (equivalent to 0.1 MOI). The final assay volume for each well was 500 µL. Each plate was incubated at 37°C for 48 hours. After incubation, the plate was thawed for 3 cycles at -70°C to rapidly isolate HSV. A titer reduction assay was performed. The Vero cells (5 × 105 cells in culture media) were seeded in each well of 6-well plates (SPL Life Sciences), maintained at 37°C in humid conditions with 5% CO2, and incubated for 48 hours. Each well of the A549 cells was serially diluted in separate 6-well plates. Then, 100 µL was removed from each well and pipetted to different wells on another 6-well plate to add overlay medium. The final assay volume reached 2 mL/well. Then, the plates were incubated at 37°C for 48 hours. The cells in each well were fixed with 500 mL of 10% formaldehyde solution and stained with 0.5% crystal violet in 20% methanol. The average number of plaques was calculated from the 10-HCPT wells, and the corresponding inhibition rate was obtained. The EC50 value was also calculated.
3.4. Evaluation of the Mechanism of Action
3.4.1. Virucidal Assay
The virucidal assay was performed, as previously described (22). Briefly, 50 μL of the HSV-1AN95 stock solution was solved with 10-HCPT (6 × EC50) and 450 μL of medium (virus-free as a negative control) at 25°C for 1 hour. Then, the treated virus suspension was further examined by titration.
3.4.2. Adsorption Assay
An adsorption inhibition test was performed to determine the effects of the hits on the virus attachment to the cell surface, as described previously (23). Briefly, the confluent A549 cell monolayers in 12-well plates were precooled for 1 hour at 4°C. Non-cytotoxic concentrations of 10-HCPT (3 × EC50) were added in triplicate. The concentrations of 10-HCPT were 0.07 µM, 0.21 µM, 0.35 µM, and 0.70 µM. Then, 200 plaque-forming units (PFU)/well of HSV-1 were added to each well immediately. All plates were then shifted at 4°C for 3 hours to allow for viral adsorption. To discard non-attached viruses, we removed the medium, and the cells were washed 3 times with sterile PBS. Then, a 2% agar overlay was added to plates, and they were finally incubated at 37°C under a humidified atmosphere containing 5% CO2 for 48 hours until the completion of viral analysis plaques in the virus control. After fixing the cells, they were stained. Besides, the inhibition percentage (I%) was computed.
3.4.3. Penetration Assay
The antiviral effects of 10-HCPT on HSV-1 host cells were assessed by a plaque reduction assay, as previously studied (24). Briefly, a 12-well plate of confluent A549 cell monolayers was precooled at 4°C for 1 hour. Then, the plate was inoculated with 200 PFU of HSV-1 at 4°C for 1 hour to uptake the virus. The cells were washed with cooled PBS and treated with 4 concentrations of triptolide (TP) and 10-HCPT at 3 × EC50 in triplicate. The concentrations of TP were 0.05 µM, 0.15 µM, 0.25 µM, and 0.75 µM, and those of 10-HCPT were 0.07 µM, 0.21 µM, 0.35 µM, and 0.70 µM. The trial plates were incubated at 4°C for 30 minutes and then immediately after incubated at 37°C to stimulate viral penetration in the presence of the test concentrations. During adsorption and treatment, the virus penetration with the concentration was inhibited by carefully doing these steps at 4°C. Following a 30-minute incubation at 37°C, the supernatants were removed, and the cells were treated under acidic conditions (low pH), inactivated HSV-1 (135 mM NaCl, 10 mM KCl, 40 mM Na-citrate pH 3.0) for 45 seconds to stop penetration and disable attached non-penetrated viruses. The citrate buffer (low pH) was discarded by washing three times with PBS, and the overlay medium was added. As described for adsorption inhibition assays, the endpoint of the experiment was done.
3.4.4. Time of Addition Assay
The efficiency of 10-HCPT (3 × EC50) as antiviral HSV-1 was evaluated by adding it to the cultured cells at various points during incubation., as previously studied (24). The experiment was performed on a 24-well plate and then inoculated with HSV-1 (200 PFU). The compounds were administered 1 hour before and after infection (1, 2, 4, 6, 12, 18, and 24 hours later). After 48 hours of infection, 3 × EC50 was loaded into the wells at a specified time. The Vero cells (5 × 105 cells) were seeded in a 6-well plate (SPL Life Sciences) and incubated with 5% CO2 for 48 hours for the titration assay. Serial dilution of each well of A549 cells was performed separately in Eppendorf tubes. Next, 100 µL of each serial dilution was poured into a 6-well plate row, and 2 mL of assay medium was added. The plates were then fixed and stained with 500 mL/well formaldehyde solution and crystal violet. The remaining infectious virus titers were further determined.
3.5. Real-Time PCR
The infected A549 cells were obtained at different time periods (1, 2, 4, 6, 12, 18, and 24 hours after infection) by doing 3 cycles of freezing and thawing after the cells were lysed. Then, viral DNA was extracted by a High Pure Viral Nucleic Acid Kit (Roche, Basel, Switzerland) and quantified by real-time PCR using an Applied Biosystem step one plus real-time PCR machine (Applied Biosystem, CA, USA). For HSV-1, the primers and probes were utilized, according to the literature (18). HSV DNA was amplified in a 20.0 μL reaction using qPCRBIO Probe Mix Hi–ROX (PCR Biosystems, London, UK). Reactions contained 10 μL of 2X qPCRBIO Probe Mix, 0.8 μL of primers, 0.8 μL of the probe, and 5 μL of the extracted DNA sample. After 2 minutes of polymerase activation at 95°C, the PCR mixture was subjected to 45 cycles at 95°C for 5 seconds and 60°C for 20 seconds.
3.6. Quantitative Reverse-Transcriptase PCR
The A549 cells were infected and treated with 10-HCPT and ACV (3 × EC50) after infection (1, 2, 4, 6, 12, 18, and 24 hours after infection). Viral RNA was extracted from the samples by a High Pure Viral RNA Kit (Roche Diagnostics, Basel, Switzerland). The purified total RNAs were then tested according to a previously published study (25) to quantify the IE genes (α4, unique short (US) 1, and unique long (UL) 54 [infected cell protein (ICP) 4, ICP22, and ICP27]), the E gene (UL29 [ICP8]), and (L) genes (UL36, UL48, UL27, UL22, and US6 [glycoprotein gB, gH, gD, VP1/2, and VP16]). These genes were assessed by qPCRBIO SyGreen 1-Step Hi-ROX-based quantitative real-time PCR (Catalog number: PB25.12, PCR Biosystems, UK) using an Applied Biosystem step one plus real-time PCR machine (Applied Biosystem, CA, USA). Quantitative real-time PCR was performed in triplicate, followed by 2 steps based on the protocol as follows: 95°C for 2 min and 45 cycles at 95°C for 5 s, at 60°C for 10 s, and 72°C for 15 s. Quantitative reverse-transcriptase PCR (RT-PCR), and primers used are presented in Table 1 (25). As an endogenous control, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used to normalize the difference in total RNA in each sample. The analysis was performed using the 2-ΔΔCT threshold cycle.
| Gene | Primer Name | Sequence (5’→3’) | |
|---|---|---|---|
| UL27 | gB | Forward | AAACCGAAAAACCCACCGCC |
| Reverse | TGTTCTCCGCCTTGATGTCC | ||
| US6 | gD | Forward | GCCCCGCTGGAACTACTATG |
| Reverse | TTATCTTCACGAGCCGCAGG | ||
| UL22 | gH | Forward | GGTTTATGGTTCGTGGGGGT |
| Reverse | CTGTCTGCTCAGTCCAGTCG | ||
| UL36 | VP1/2 | Forward | CGGGTCAAAAAGGTATGCGG |
| Reverse | TGTCGTACACGCTCCTAACC | ||
| UL48 | VP16 | Forward | TTTGACCCGCGAGATCCTAT |
| Reverse | GCTCCGTTGACGAACATGAA | ||
| α4 | ICP4 | Forward | CGACACGGATCCACGACCC |
| Reverse | GATCCCCCTCCCGCGCTTCGTCCG | ||
| UL29 | ICP8 | Forward | CGACAGTAACGCCAGAAG |
| Reverse | GGAGACAAAGCCCAAGAC | ||
| UL54 | ICP27 | Forward | ATGTGCATCCACCACAACCT |
| Reverse | TCCTTAATGTCCGCCAGACG | ||
| US1 | ICP22 | Forward | CGCCGCAGAAGACCGCAAGT |
| Reverse | TGTCGCTGCACGGATAGGG | ||
| GAPDH | GAPDH | Forward | GGTGGTCTCCTCTGACTTCAACA |
| Reverse | GTTGCTGTAGCCAAATTCGTTGT | ||
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; US, unique short ;UL, unique long; ICP, infected cell protein; VP, viral protein; g, glycoprotein.
3.7. SDS-PAGE and Immunoblotting Assays
By transferring to a transfer buffer (0.248 M Tris-HCl, pH 8.8, 1.92 M glycine, 20% methanol) and using a 10% polyacrylamide gel run at 150 V (MiniProtean 3; Bio-Rad), SDS-PAGE was used to evaluate the samples (15 µL each). The nitrocellulose membrane was added to the bottom of the gel. To get the proteins, the gel was run at 60 V for 3 hours. Using 5% skim milk dissolved in Tris buffer saline containing 0.1% Tween 20 (TBST) for 50 minutes at ambient temperature, the binding sites that were nonspecific on the membranes were blocked. Using proper antibodies (HA021 and H1A027), blots were incubated.
The anti-HSV-1/2 gD monoclonal antibody (Ref. MAB-13574, Abnova) diluted 1:2000, and anti-HSV-1 ICP0 and ICP4 monoclonal antibodies (Ref. MAB-13583 and MAB-13584, Abnova) diluted 1:2000. Also, 3G2, the anti-GAPDH monoclonal antibody (H00002597-M01, Abnova), was incubated overnight at 4°C. The blots were incubated with appropriate Goat Anti-Mouse IgG (H&L) secondary antibody (Peroxidase) or mouse anti-mouse secondary antibodies (PAB0096, Abnova, Canada; 1: 1000) at room temperature for 1 hour by washing three times with TBST. Using horseradish peroxidase-conjugated secondary antibodies, membranes were incubated for 2 hours at room temperature. Using GeneTools software (SynGene, UK), the intensities of bands were calculated.
3.8. Statistics
The selectivity indices (SI = CC50/EC50), dose-inhibitory rate curve, and dose-viability curve regression analyses were calculated using GraphPad Prism version 5 (GraphPad Software Inc., La Jolla, CA). The treatment groups were compared for differences using this software with analysis of variance (ANOVA). P values less than 0.05 were considered statistically significant.
4. Results
4.1. Identification of 10-HCPT as Inhibitors of HSV Infection
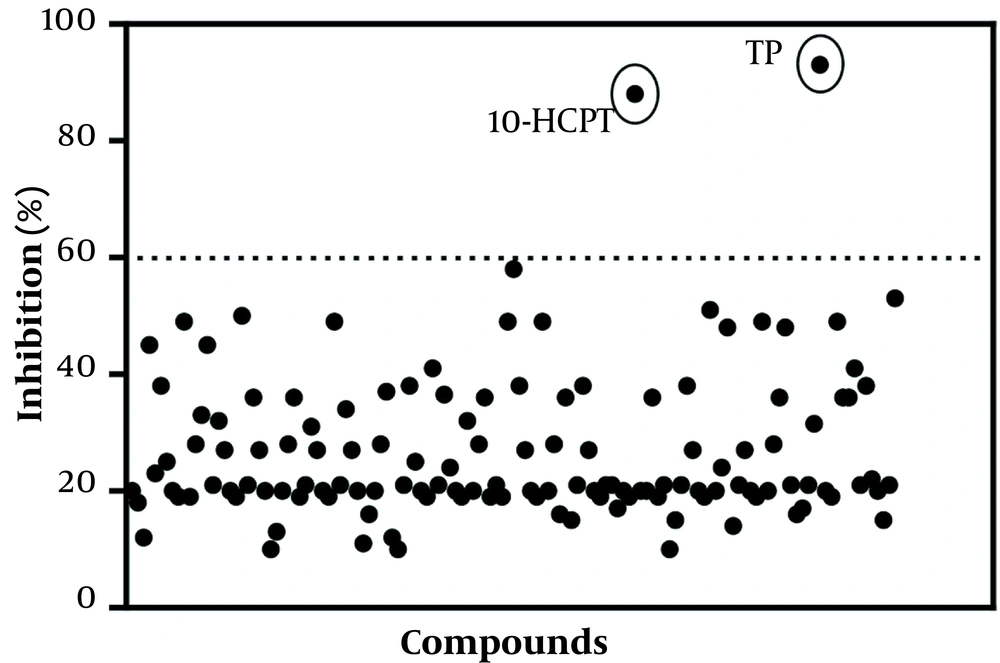
A set of natural products consisting of 133 compounds was screened for anti-HSV effects in 2 concentrations of 5 µM and 30 µM by a CPE-based assay in a 96-well plate. Most effective compounds were selected with a cutoff of 60% CPE inhibition. Two primary compounds obtained had over 80% CPE inhibition; 10-HCPT among compounds inhibited the HSV-1-induced CPE in a dose-dependent manner with no considerable cytotoxicity (Figure 1). The selected compounds were solved in DMSO and then carried out twice in secondary assays, comprising the same CPE-based assay for initial screening to ensure the antiviral activities of such compounds. After that, the A549 cells were infected with 0.1 MOI of HSV-1 in the presence of this compound. Conventional plaque assays on Vero cells were employed to quantify the amount of HSV-1 produced by the A549 cells. To our surprise, dose-dependent inhibition of infection by 10-HCPT and other selected compounds with plaque assays was confirmed for a concentration of 5 µM. The compound displayed maximum antiviral activity at this concentration from the dose-response curves. All such findings support the inhibition of HSV-1 infection by the primary hits.
Identification of triptolide and (S)-10-hydroxycamptothecin as inhibitors of herpes simplex virus type 1 infection. Primary screening of 133 compounds from the natural product library in a single dose of 5 μM. Inhibitory effects were calculated as percentage cytopathogenic effect inhibition. Hits were selected with a cutoff of 60% cytopathogenic effect inhibition (dotted line).
4.2. Cytotoxicity of 10-HCPT
The cell viability was assessed by a WST-1 assay. The CC50 of 10-HCPT on A549 cells was 3.596 μM. The CC50 of this compound on PRK cells was 6.111 μM (Table 2).
Abbreviations: CC50, 50% cytotoxic concentration; EC50, half maximal effective concentration.
a Values obtained by the plaque reduction assay.
b Values obtained by WST-1 on A549 cells.
c Values obtained by WST-1 on primary cells.
4.3. Plaque Reduction Assays and Determination of the EC50
The antiviral effect of 10-HCPT on HSV-1 infectivity was evaluated using a plaque reduction assay initially performed in vitro on PRK and A549 cells. Then, 5 selected concentrations were analyzed 3 times in 2 secondary assays, used for the primary screening and a parallel EC50 assay. The results were considered as a percentage of reduced plaques detected in the treated natural product compared to DMSO. Afterward, the plaques were counted on Vero cells. The results showed that each 10-HCPT could inhibit HSV-1 with a value of 0.07 μM as EC50 (Table 2). A WST-1 assay determined the CC50 value to determine the SI of the selected compound. As revealed in Table 2, the SI was 51.37 for 10-HCPT. In addition, the SI was 330.15 in PRK cells, determined by regression analysis and dose-survival rate curve.
4.4. Evaluation of the Possible Antiviral Mechanism
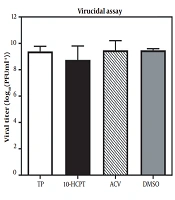
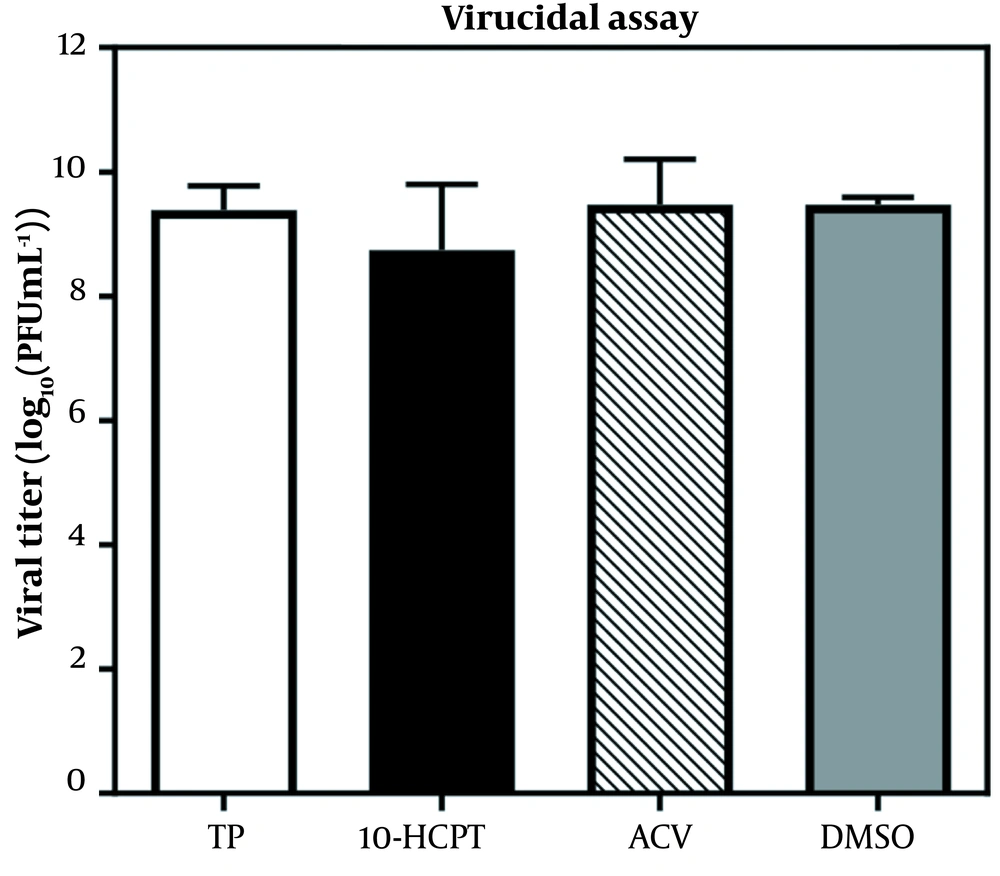
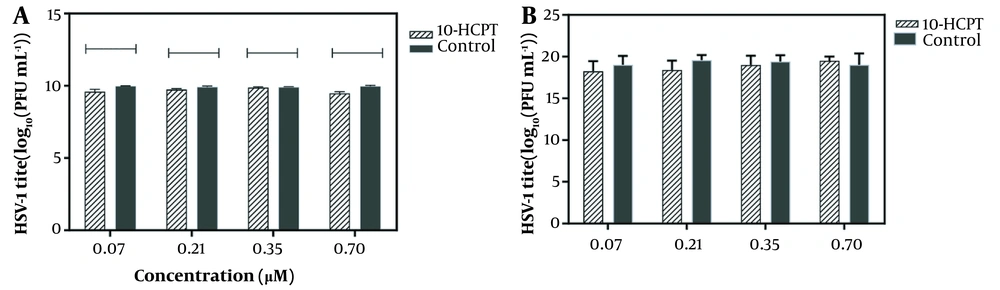
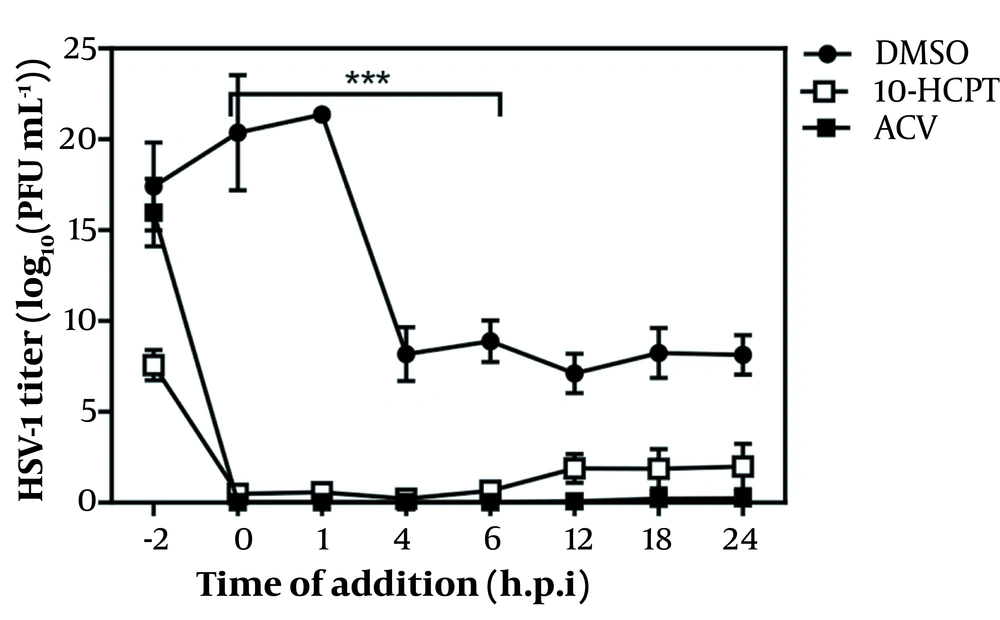
Before monolayer infection, infectious HSV-1 particles were incubated with a hit to investigate whether selected 10-HCPT could directly inactivate viral particles. Figure 2 shows that this compound was not virus-killing. We investigated the antiviral activity of 10-HCPT on cell invasion or interference with the early stages of viral production, including viral adsorption and penetration into Vero cells. The adsorption and permeation assays showed that 10-HCPT did not significantly reduce virus yield compared to DMSO (Figure 3A and B). After this, the adsorption and permeation assays showed that this compound could not kill HSV-1 directly and block the invasion of HSV-1 into A549 cells. A time-of-addition test was performed to specify the probable phases of the viral replication cycle targeted by 10-HCPT. As shown in Figure 4, the virus yield titration was perceived as high when this compound was introduced, with a reduction before 8 hours after infection. The levels of the virus yield titration in ACV-treated cultures were almost the same as the baseline during the experiment compared to the inoculum virus.
The mechanism study of (S)-10-hydroxycamptothecin-mediated inhibition of herpes simplex virus type 1. The virucidal efficacy of (S)-10-hydroxycamptothecin. Herpes simplex virus type 1 was mixed with acyclovir, dimethyl sulfoxide (0.10%; consisting of dimethyl sulfoxide, virus, and cell), and (S)-10-hydroxycamptothecin (6 × EC50) and then incubated at 25°C (room temperature) for 1 hour. The virus titers were indicated by a plaque reduction assay. The data were obtained by repeated experiments. Error bars demonstrate SDs from 3 times independent experiments.
The effect of (S)-10-hydroxycamptothecin on the virus, A, adsorption; and B, penetration steps of entry. This procedure was described in the Methods Section. The data represent the results of independent experiments performed 3 times. Statistically, the significant value between the treated group and dimethyl sulfoxide-treated group as control was evaluated by a Student t test as follows: ns, ***, P < 0.001 or ****, P < 0.0001.
Time-of-addition study. The A549 cells infected with herpes simplex virus type 1 at MOI of 0.1, acyclovir (0.01 μg/mL), and (S)-10-hydroxycamptothecin (3 × EC50) were added to the cells at the demonstrated study time points of infection (before and after infection). The infected cells were lysed by doing 3 steps of freezing and thawing. The treated viruses in specific time courses were further evaluated by titration on the Vero cell. The data were assessed by virus yield reduction of cell lysates and represent the mean of a 3-independent experiment (± SE). (∗∗∗) demonstrated significant statistical differences between the treated groups (P < 0.0001). Analysis of variance/Dunnett tests were carried out as appropriate.
4.5. Yields of Viral Nucleic Acid in the Presence of 10-HCPT
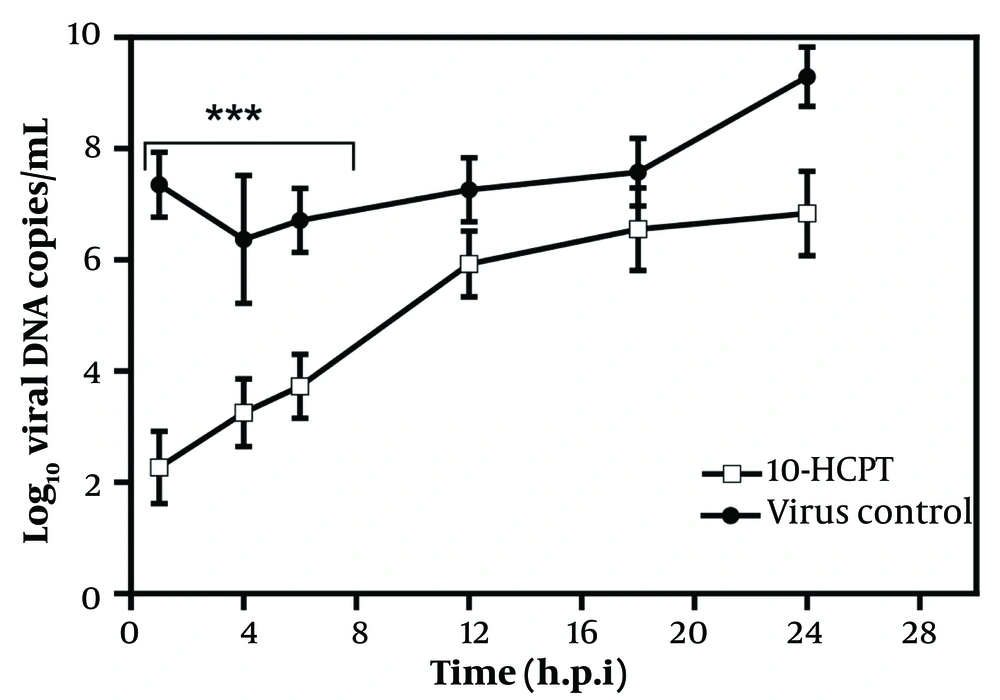
During investigating the antiviral property of 10-HCPT on HSV-1 DNA yields, periodic examinations were performed with or without this added compound 1 hour before infection and 1, 2, 4, 8, 14, and 24 hours after infection after the penetration phase, and uncoating processes were performed (Figure 5). These analyses showed that 10-HCPT significantly inhibited HSV-1 DNA replication if introduced up to 8 hours after infection.
The real-time polymerase chain reaction was performed to evaluate the number of herpes simplex virus type 1 DNA copies after treating with (S)-10-hydroxycamptothecin (a), in the presence or absence of (S)-10-hydroxycamptothecin, compared to the dimethyl sulfoxide control (consisting of the cells, dimethyl sulfoxide, and virus). Data are presented as mean values of triplicate with its SD. Significant differences were found among herpes simplex virus type 1 DNA levels up to 8 hours after infection from (S)-10-hydroxycamptothecin concerning dimethyl sulfoxide controls (consisting of the cells, dimethyl sulfoxide, and virus). (∗∗∗) indicates significant statistical differences between the treated groups and the dimethyl sulfoxide control (P < 0.0001). Analysis of variance/Dunnett tests were carried out as appropriate.
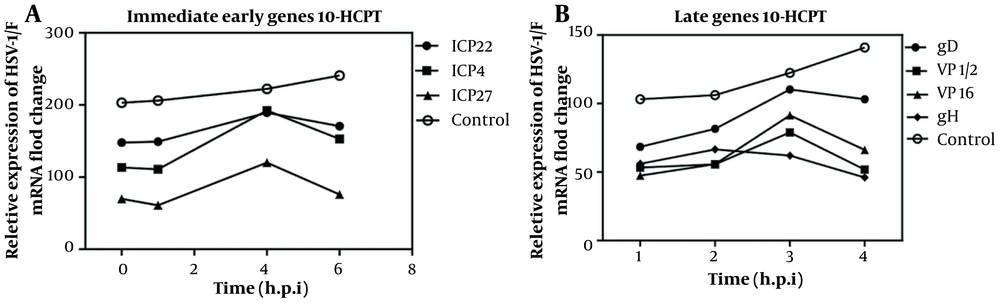
4.6. 10-HCPT Effect on the Expression of HSV-1 IE, E, and L late Genes
The messenger RNA (mRNA) levels of ICP4, ICP22, and ICP27 genes up to 6 hours after infection in the 10-HCPT treatment group were insignificant compared to those of the DMSO group. Furthermore, the expression pattern of the L gene (glycoproteins gD, gH, gB, VP1/2, and VP16) in the presence of 10-HCPT was not similar to that of the DMSO group 4 hours after infection (Figure 6A and B). Similarly, the mRNA level of ICP8 as the E gene in the treatment group was not significantly different after 12 hours after infection compared to DMSO (data not shown). ICP4, ICP22, and ICP27 genes have the most significant effect on early and late promoters as essential IE regulatory proteins. In addition, gD, which functions as the L protein, is a coat glycoprotein that binds to potential invading receptors in host cells. This glycoprotein uses a fusion mechanism consisting of gB and gH /gL to stimulate viral fusion with the host membrane (1).
Effects of (S)-10-hydroxycamptothecin (3 × EC50) on the expression of herpes simplex virus type 1 genes (immediate-early, and late). A, A549 cells were infected with herpes simplex virus type 1 at 0.1 MOI and then treated with or without 3 × EC50 of each compound. RNA was extracted at specified intervals, and a quantitative real-time polymerase chain reaction was performed using messenger RNA-specific primers. The gene transcription level at each time point was initially normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and then compared with the virus control group into 24 hours after infection.
5. Discussion
Drug-resistant strains have become a global health problem. Eliminating HSV infections in humans requires new strategies (26). Because of the high incidence of HSV infection worldwide, it is unlikely to decrease very soon (8). Increasing the number of immunocompromised patients and using appropriate treatments can exacerbate the problems associated with drug-resistant HSV. Despite existing effective standard therapies and low resistance rates, new therapies need to be developed to avoid future HSV epidemics (10). As can be seen in the literature, natural agents rather than synthetic agents have proven to be the most effective sources of new drugs, especially antibacterial and antineoplastic agents (11, 13, 27). So far, many studies were carried out on products, such as crude extracts, fractions, and pure compounds that were either isolated from plants, animals, microorganisms, or marine life for their antiviral effect on HSV. Therefore, these potential anti-HSV agents need to be studied as alternatives to nucleoside analogs to promote therapeutic efficacy (14).
From 1981 to 2010, approximately 34% of the US Food and Drug Administration (FDA)-approved natural product fractions were either natural or the corresponding direct derivative (15). To identify new inhibitors of HSV-1, we first screened a natural product library containing 133 compounds. In this study, we identified 10-HCPT among 2 selected compounds as inhibitors of HSV-1 infection through a screening assay. The inhibition hit rate was higher than the previous FDA-approved drug library screening (0.23%), the low cutoff for 30% CPE inhibition (28). A molecule called 10-HCPT, a CPT, specifically blocks topoisomerase I that breaks and leaves the DNA strand during the replication stage. A few studies have reported that CPT inhibits the replication and packaging of double-stranded and single-stranded DNA containing HSV-2, adenovirus viruses, papovaviruses, and autonomous parvovirus (29-31).
As revealed, the natural product library possessed HSV-1 antiviral activity, but TP and 10-HCPT showed a significant inhibitory effect. We previously found that TP presented a significant inhibitory effect on HSV-1 plaque formation with an EC50 of 0.05. Moreover, the time-of-addition assay suggested that TP had viral inhibitory effects when added 8 hours after infection with EC50 of 0.07 µM in A549 cells (1, 32, 33). As shown in the data, 10-HCPT was highly effective in the inhibition of virus titration on A549 and PRK cells with EC50 of 0.068. The EC50 value of ACV was 0.01-μg/mL concentration on A549 cells when used as a gold standard anti-HSV. It is noteworthy that the HSV-1 isolated in this study was sensitive to ACV, according to our previous experiment (17).
The CC50 value by the WST-1 assay to calculate the SI of 10-HCPT was 51.37 (Table 2). According to Prichard et al., a SI value of > 1 was sufficient to show antiviral activity (16). However, a higher SI value is needed to suggest a safe antiviral therapeutic range due to the significant difference between cytotoxic and antiviral concentrations. The antiviral activity of 10-HCPT was dose-dependently lower than the cytotoxic concentration, indicating that some mechanism rather than cytotoxicity mediated it. Liu et al. reported that the 10-hydroxy derivative of CPT showed the highest anti-HSV 2 activity, with an EC50 value of 3.33 mg/mL and a SI value of 12.95 (29).
We used a time-of-addition assay to further characterize the inhibitory effect of 3 × EC50 μM 10-HCPT. We assessed viral infectivity during different HSV-1 replication cycle steps in a time-dependent manner on A549 cells. The plaque reduction assays showed that 10-HCPT had effects in IE and L stages between 4 and 8 hours of viral replication. Thus, complementary assays targeting early virus production steps were also performed. The virus inactivation, attachment, and penetration assays showed that 10-HCPT could not affect viral attachment and penetrate cells at 0.01 MOI. Thus, 10-HCPT could not stop HSV-1 from attaching and being absorbed into the cells since the entry of herpesviruses into their target cells is complex at many levels depending on viral MOI and the kind of cells.
A virucidal assay was performed to indicate the inactivating capacity of 10-HCPT on virions. Our results showed that it was not effective in the inhibition of the virus compared to the DMSO group. It is generally admitted that a good antiviral drug must reduce viral infection by 2 log10 (99% inactive) or more (34). In other studies, compounds (25, 35) had no effect on adsorption and penetration stages like 10-HCPT. Interestingly, the time-of-addition assay results indicated that the critical time for the inhibition of 10-HCPT on HSV-1 replication was up to 8 hours after infection. The viral DNA replication, L gene transcription, and encapsidation of viral DNA occur at the HSV replication site (36). On the other hand, 10-HCPT had HSV-1 replication inhibition effects. Using real-time PCR, we performed a kinetic study to assess the 10-HCPT effect on viral DNA replication. The DNA concentration in cultures containing 10-HCPT decreased in comparison with our DMSO. Hence, 10-HCPT can inhibit the expression of viral genes in the IE and L stages, respectively. However, the ICP4 protein expression was not impeded in the 10-HCPT-treated group, suggesting that HSV replication formation compartments with other genes in the IE and L stage may be inhibited in the presence of 10-HCPT. In this case, HSV-1 is actively replicated in the nucleus by entering the host cells.
5.1. Conclusions
Overall, 10-HCPT demonstrated anti-HSV activity on HSV-1. Their dose-dependent antiviral activity showed that specific cellular components might mediate their function rather than cytotoxicity. Unfortunately, to date, studies on those aspects related to the antiviral effect of this compound are limited. Further studies focusing on the activity of anti-HSV-1 mechanisms in vivo are necessary.