1. Background
Coronavirus disease 2019 (COVID-19) is a pandemic infectious disease organed by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). Clinical in patients with COVID-19 is mainly associated with hypercytokinemia resulting from extreme overproduction of proinflammatory cytokines (2). In reply to SARS-CoV-2, antigen-presenting cells (APCs) such as macrophages and dendritic cells can present antigen fragments to naïve CD4+ T cells, and activated APCs secrete polarizing cytokines such as IL-6, TGF-β, and IL-23 (2). IL-6 then binds to its receptor via signal transducer and activator of transcription 3 (STAT3), which upregulates the expression of retinoic acid receptor-related orphan receptor-gamma (RORγ), leading to polarization, maturation, and enlargement of CD4+ T cells into Th17 cells. Activated Th17 cells produce signature cytokines such as IL-17A, IL-17F, IL-21, and IL-22 in response to polarizing cytokines secondary to viral infection in the lung alveoli. In short, IL-17A is a cytokine that may be associated with IL-6, especially in the context of viral infection (2-5). However, IL-17A synthesis is not solely dependent on IL-6 (6).
IL-17 is an important mediator of pulmonary inflammation among the many cytokines-related cytokine release syndromes. IL-17 activates many signaling pathways, producing many other cytokines and chemokines by a wide range of alveolar cell types (4, 7). The immunopathologic effect of IL-17A results in IL-6-mediated fibroblast activation and subsequent abnormal collagen accumulation and pulmonary fibrosis. IL-8 can be produced by stimulating fibroblasts, leading to neutrophil chemotaxis (4). Prostaglandin E2 (PGE2) increases vascular permeability and neutrophil infiltration, which is responsible for pleural effusion and alveolar edema. In addition, IL-17A has also been reported to increase platelet activation and modulate arterial thrombus formation in vivo via the extracellular signal-regulated kinase 2 (ERK-2) signaling pathway (4, 7). In summary, IL-17A may mediate numerous immunopathologic effects secondary to cytokine release syndrome during SARS-CoV-2 infection. Therefore, it is believed that blocking IL-17A could potentially mitigate the abnormal immune response to COVID-19 and lower the rate of mortality associated with acute respiratory distress syndrome (ARDS) (3).
2. Objectives
3. Methods
3.1. Study Design
Patients with clinical signs of COVID-19 and typical high-resolution computed tomography (HRCT) findings (peripherally distributed ground-glass opacity, subsegmental consolidation, and crazy-paving pattern) and those with atypical radiological findings but consistent clinical presentation were hospitalized. Patients presenting with an initial respiratory rate higher than 30 breaths/min, oxygen saturation (SpO2) lower than 90%, and more than 50% lung infiltration were admitted to the intensive care unit; others were admitted to the ward. Demographic data identified as mortality risk factors and clinical and radiological findings on the day of hospital admission and daily C-reactive protein (CRP), ferritin, albumin, alanine aminotransferase (ALT), D-dimer levels, and neutrophil-to-lymphocyte ratio (NLR) were recorded.
3.2. Study Group
The study included 202 participants: 152 COVID-19 patients and 40 controls. The control group (n = 40) consisted of asymptomatic healthcare professionals with negative SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) results. The patients were divided into two groups, survivors (n = 86) and non-survivors (n = 66). The 152 COVID-19 patients were also classified according to disease severity at admission: 50 (32.9%) had moderate, 48 (31.6%) had severe, and 54 (35.5%) had a critical disease.
3.3. Inclusion and Exclusion Criteria
Patients aged 18 years or older who tested positive for SARS-CoV-2 in respiratory tract samples by RT-PCR in laboratories authorized by the Turkish Ministry of Health were included. IL-17A was evaluated at baseline and on day seven. Those whose one of these two assessments were missing or died within the first seven days were not included in the study.
3.4. Definitions
Fever was defined as a body temperature of 38.3°C or higher, hypoxia as SpO2 of 93% or lower, tachycardia as a pulse of 100 beats/min or higher, and hypotension as arterial blood pressure of 90/60 mmHg or lower. Disease severity was classified according to the World Health Organization criteria and the patients' findings at the presentation (1).
3.5. IL-17A Measurement
Samples were collected in anticoagulant-free tubes and left at room temperature for 30 minutes. Afterward, it was centrifuged at 1000 × g for 15 minutes at 4°C as per the instructions provided with the enzyme-linked immunoassay (ELISA) kit. The resulting serum samples were transferred to Eppendorf tubes and stored at -80°C until analysis. Serum IL-17A levels were measured using the Human Interleukin 17A ELISA Kit (Bioassay Technology Laboratory, China, Cat. No: E0047Hu) following the manufacturer's protocol. The assay provided results in ng/L and had a lower sensitivity limit of 2.38 ng/L.
3.6. Statistical Analysis
Data from 152 patients in the study cohort and 40 controls were included in the analysis. Quantitative variables were tested for normal distribution with the Kolmogorov-Smirnov test (P > 0.05). Categorical variables were analyzed using the chi-square or Fisher's exact test. Continuous variables were compared between the survivor and non-survivor groups using Student's t-test or Mann-Whitney U test as appropriate. Receiver operating characteristic (ROC) curves were generated to demonstrate the predictive ability of the variables for mortality. The Youden index was used to determine optimal cut-off values. The area under the curve (AUC) was calculated to determine the diagnostic power of risk factors. The Kaplan-Meier method compared cumulative survival curves and their differences between categorized groups with log-rank tests. Between-group differences with P < 0.05 in log-rank tests were considered statistically significant.
4. Results
4.1. Baseline Characteristics
The median age of the COVID-19 patients included in the study was 65 years (range 19 - 89), and 80 (52.6%) were men. A comparison of the patients’ basic characteristics according to mortality is presented in Table 1. Non-survivors were significantly older than survivors and had a higher frequency of hypertension, diabetes mellitus, coronary artery disease, and chronic obstructive pulmonary disease. Approximately half of the patients had a fever at presentation. Complaints of cough, fever, dyspnea and hypoxia, hypotension, and tachycardia at presentation were more common among non-survivors. On lung CT imaging, consolidation, crazy-paving pattern, pulmonary embolism, and pleural effusion were observed more frequently in non-surviving patients (Table 1).
| Variables | All Patients (n = 152) | Survivors (n = 86) | Nonsurvivors (n = 66) | P b |
|---|---|---|---|---|
| Demographic data | ||||
| Age (y), median (range) | 65 (19 - 89) | 61 (19 - 86) | 72 (25 - 89) | < 0.001 |
| Gender (male) | 80 (52.6) | 46 (53.5) | 34 (51.5) | 0.809 |
| Comorbid diseases | ||||
| Hypertension | 92 (60.5) | 38 (44.2) | 54 (81.8) | < 0.001 |
| Diabetes mellitus | 55 (36.2) | 25 (29.1) | 30 (45.5) | 0.037 |
| CAD | 42 (27.6) | 13 (15.1) | 29 (43.9) | < 0.001 |
| COPD | 32 (21.1) | 11 (12.8) | 21 (31.8) | 0.004 |
| CKD | 8 (5.3) | 2 (2.3) | 6 (9.4) | 0.057 |
| Malignancy | 8 (5.3) | 4 (4.7) | 4 (6.1) | 0.486 |
| Asthma | 6 (3.9) | 3 (3.5) | 3 (4.5) | 0.528 |
| Clinical symptoms | ||||
| Cough | 119 (78.3) | 60 (69.8) | 59 (89.4) | 0.004 |
| Malaise | 116 (76.3) | 69 (80.2) | 47 (71.2) | 0.195 |
| Dyspnea | 110 (72.4) | 46 (53.5) | 64 (97.0) | < 0.001 |
| Myalgia | 99 (65.1) | 56 (65.1) | 43 (65.2) | 0.996 |
| Fever | 88 (57.9) | 40 (46.5) | 48 (72.7) | 0.001 |
| Anorexia | 73 (48.0) | 38 (44.2) | 35 (53.0) | 0.279 |
| Headache | 42 (27.6) | 19 (22.1) | 23 (34.8) | 0.081 |
| Nausea | 41 (27.0) | 23 (26.7) | 18 (27.3) | 0.942 |
| Sore throat | 26 (17.1) | 18 (20.9) | 8 (12.1) | 0.153 |
| Diarrhea | 17 (11.2) | 7 (8.1) | 10 (15.2) | 0.174 |
| Loss of taste and smell | 3 (2.0) | 2 (2.3) | 1 (1.5) | 0.722 |
| Initial vital signs | ||||
| Hypoxia | 102 (67.1) | 39 (45.3) | 63 (95.5) | < 0.001 |
| Hypotension | 14 (9.6) | 1 (1.2) | 13 (21.7) | < 0.001 |
| Tachycardia | 33 (21.7) | 3 (3.5) | 30 (45.5) | < 0.001 |
| Initial lung tomography findings | ||||
| Ground-glass opacities | 140 (92.1) | 78 (90.7) | 62 (93.9) | 0.463 |
| Pleural effusion | 18 (11.8) | 2 (2.3) | 16 (24.6) | < 0.001 |
| Pulmonary embolism | 9 (5.9) | - | 9 (13.6) | < 0.001 |
| Consolidation | 66 (43.4) | 21 (24.4) | 45 (68.2) | < 0.001 |
| Crazy-paving pattern | 59 (38.8) | 22 (25.6) | 37 (56.1) | < 0.001 |
| Interlobular septal thickening | 30 (19.7) | 14 (16.3) | 16 (24.2) | 0.221 |
Abbreviations: CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease.
a Values are expressed as No. (%) unless otherwise indicated.
b Chi-square and Mann-Whitney U test.
4.2. Laboratory Results
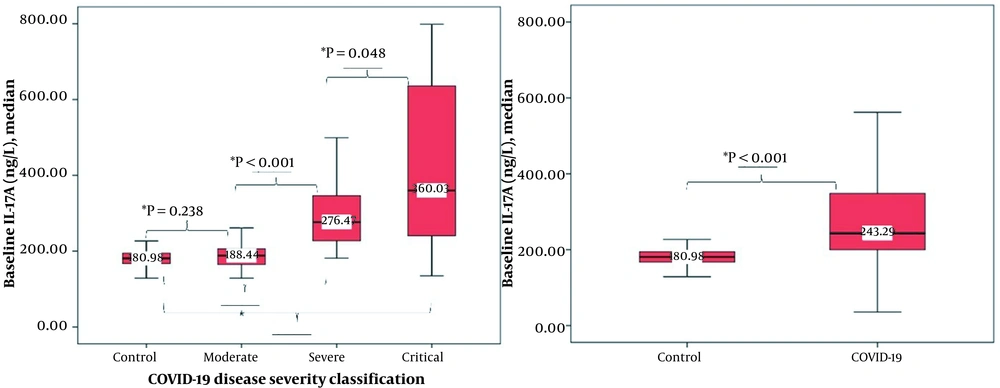
The results of the patient's laboratory tests at admission and on day seven are presented in Table 2. IL-17A, CRP, ferritin, creatinine, D-dimer, and NLR were significantly higher, and albumin was lower in non-survivors. Comparisons of IL-17A levels between the control group and the COVID-19 patient group and disease severity subgroups are shown in Figure 1. IL-17 levels were significantly higher in COVID-19 patients than in controls and were positively associated with disease severity (moderate vs. severe, P < 0.001; severe vs. critical, P = 0.048). There was no significant difference between controls and patients with moderate disease (P = 0.238). However, there was a significant difference between the control group and severe and critical patients (P < 0.001).
| Baseline and Day 7 Laboratory Findings | All Patients (n = 152) | Survivors (n = 86) | Nonsurvivors (n = 66) | P b |
|---|---|---|---|---|
| IL-17A, day 0 | 213.19 (35.67 - 798.68) | 212.42 (35.67 - 650.17) | 277.81 (135.01 - 798.68) | < 0.001 |
| IL-17A, day 7 | 263.22 (128.70 - 798.68) | 200.93 (128.70 - 650.17) | 348.87 (129.16 - 798.68) | < 0.001 |
| CRP, day 0 (mg/L) | 67 (3 - 306) | 40 (3 - 199) | 113 (5 - 306) | < 0.001 |
| CRP, day 7 (mg/L) | 9.3 (3 - 388) | 4 (3 - 161) | 72.5 (3 - 388) | < 0.001 |
| Ferritin, day 0 (ng/mL) | 418 (3.5 - 3762) | 257 (3.5 - 2287) | 1133 (12 - 3762) | < 0.001 |
| Ferritin, day 7 (ng/mL) | 392 (24 - 1650) | 243 (24 - 1650) | 1200 (71 - 1650) | < 0.001 |
| Albumin, day 0 (g/L) | 39 (18 - 47) | 42 (31 - 47) | 35 (18 - 46) | < 0.001 |
| Albumin, day 7 (g/L) | 33.5 (15 - 46) | 37 (27 - 46) | 29 (15 - 33) | < 0.001 |
| ALT, day 0 (U/L) | 32 (9 - 297) | 31 (13 - 156) | 33 (9 - 297) | 0.824 |
| ALT, day 7 (U/L) | 40.5 (9 - 679) | 39 (9 - 226) | 45 (14 - 679) | 0.341 |
| D-dimer, day 0 (ng/mL) | 856 (190 - 35200) | 534 (190 - 16279) | 1765 (190 - 35200) | < 0.001 |
| D-dimer, day 7 (ng/mL) | 732 (190 - 35200) | 453 (190 - 7106) | 2273 (190 - 35200) | < 0.001 |
| NRL, day 0 | 3.35 (0.58 - 39.75) | 2.54 (0.58 - 16.01) | 9.13 (0.73 - 39.75) | < 0.001 |
| NRL, day 7 | 9.49 (1.16 - 156.53) | 6.00 (1.16 - 31.77) | 15.62 (1.27 - 156.53) | < 0.001 |
Abbreviations: CRP, C-reactive protein; ALT, alanine aminotransferase; IL-17A, interleukin 17A; NRL, neutrophil-to-lymphocyte ratio.
a Values are expressed as median (range).
b Mann-Whitney U test.
4.3. The Predictive Power of Baseline Laboratory Values
The determined cut-off values, predictive mortality power, specificity, and sensitivity of IL-17A and selected routine biomarkers measured at hospital admission are presented in Table 3. At a cut-off value of 210.25 ng/mL, IL-17A had an area under the curve of 0.792 and predicted mortality with 84.8% sensitivity and 63.5% specificity. NRL had the best predictive power for mortality.
| Variables | Cut-Off | AUC (95% CI) | Sensitivity (%) | Specificity (%) | P |
|---|---|---|---|---|---|
| IL-17A | 210.25 ng/L | 0.792 (0.727 - 0.857) | 84.8 | 63.5 | < 0.001 |
| CRP | 118.5 mg/L | 0.765 (0.689 - 0.840) | 50.0 | 93.0 | < 0.001 |
| Ferritin | 555 ng/mL | 0.797 (0.723 - 0.872) | 69.7 | 82.6 | < 0.001 |
| Albumin | 39 g/L | 0.835 (0.767 - 0.903) | 70.9 | 87.9 | < 0.001 |
| D-dimer | 628 ng/mL | 0.816 (0.748 - 0.883) | 90.9 | 58.1 | < 0.001 |
| NRL | 6.00 | 0.857 (0.794 - 0.920) | 66.7 | 95.3 | < 0.001 |
Abbreviations: CI, confidence interval; CRP, C-reactive protein; IL-17A, interleukin 17A.
4.4. Day-7 Changes and Predictive Mortality Power of Laboratory Parameters
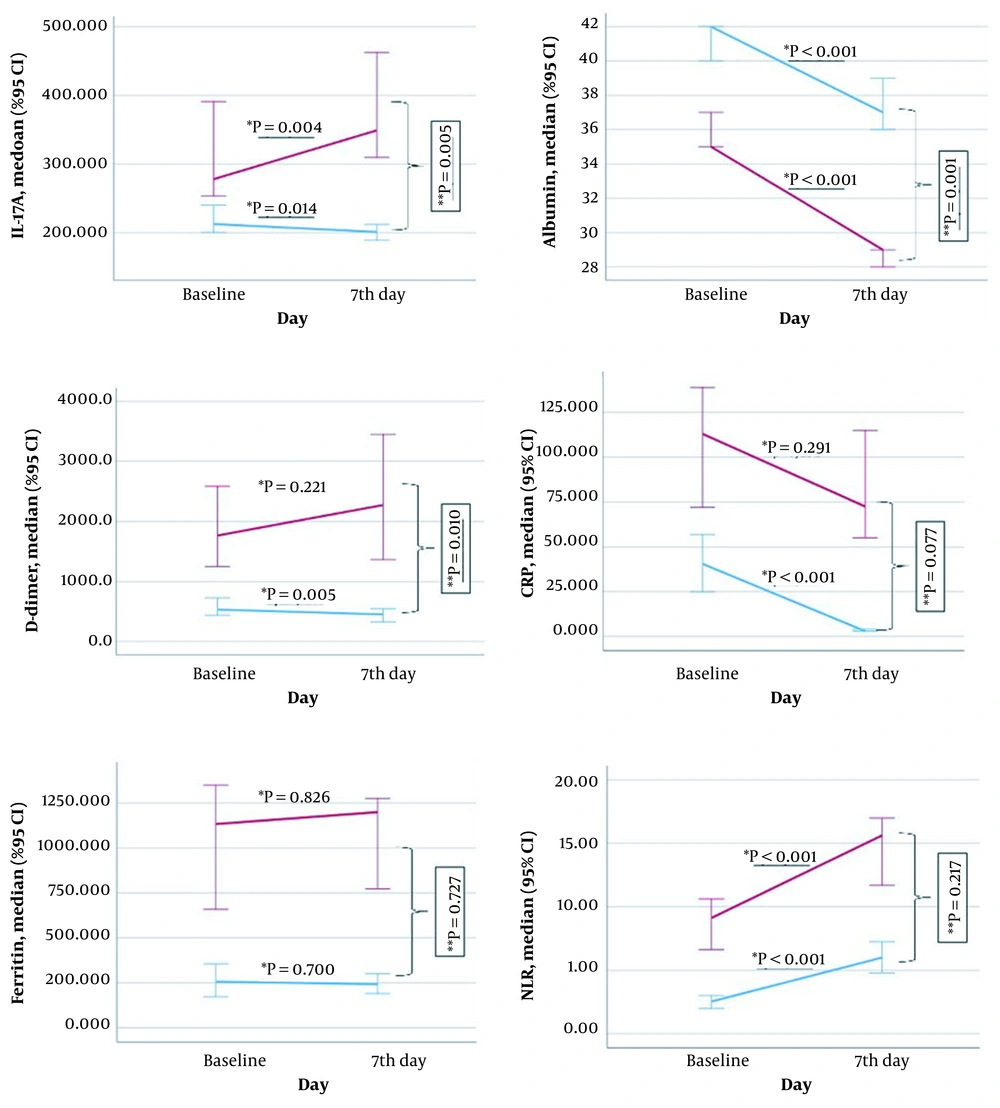
The comparison of changes in levels of biomarkers from baseline to day seven according to mortality is shown in Figure 2. Compared to baseline, IL-17A values on day seven were significantly increased in non-survivors (P = 0.004) and decreased in survivors (P = 0.014). The change in median IL-17A value from baseline to day 7 differed significantly between survivors and non-survivors (non-survivors: 41.47, range -316.65 - 458.42, survivors: -11.66, range -458.42 - 178.54; P = 0.005). CRP levels fell significantly on day seven compared to baseline only in surviving patients (P < 0.001). The change in median CRP value from baseline to day 7 showed no association with mortality (non-survivors: -16, range -236 - 345, survivors: -32, range -196 - 158; P = 0.077).
Albumin was significantly decreased on day seven compared to initial values in survival and non-surviving patients (P < 0.001). This change differed significantly according to mortality (non-survivors: -7, range -5 - 7, survivors: -3, range -4 - 6; P = 0.001). Ferritin levels did not change significantly from baseline to day 7 in survivors or non-survivors (P = 0.826 and P = 0.700, respectively), and changes in ferritin levels did not differ significantly according to mortality (non-survivors: 0, range -2112 - 1474, non-survivors: -1, range -1550 - 1280; P = 0.727). D-dimer values on day 7 showed a nonsignificant increase in non-surviving patients (P = 0.221) and were significantly decreased in survivors (P = 0.005) compared to baseline values. The change in median D-dimer level from baseline to day 7 differed significantly according to mortality (non-survivors: 148, range -30322 - 30520, survivors: -74.5, range -15646 - 6616; P = 0.010). NRL values increased significantly from baseline to day 7 in all patients (P < 0.001 for both groups). These changes were statistically similar in both survivors (3.25, range -10.90 - 28.83) and non-survivors (4.17, range -16.88 - 116.78) (P = 0.217).
The determined cut-off values, predictive power, specificity, and sensitivity of day-7 changes in IL-17A and selected routine biomarkers for predicting mortality are presented in Table 4. Biomarkers that significantly changed from baseline to day 7 had similar predictive power for mortality. An increase in IL-17A of 26.18 ng/L or more from baseline to day 7 had a predictive mortality power of 0.634 (0.532 - 0.736), a sensitivity of 56.1%, and a specificity of 84.9%.
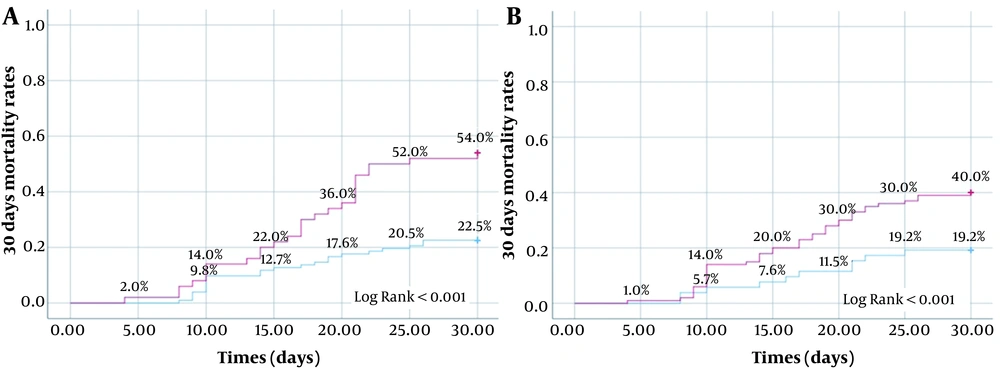
An increase in D-dimer of 771 ng/mL or more from baseline to day 7 predicted mortality with high specificity (97.7%). Figure 3 shows the mortality rates in the first month for patients whose change in IL-17A on day seven and baseline IL-17A levels were above and below the determined cut-off values. The optimum cut-off value for a 7-day change in IL-17A for predicting mortality was 26.18 ng/L. The 30-day mortality rates for patients above and below this cut-off value were 54.0% and 22.5%, respectively. Survival at 30 days was significantly more common for patients whose IL-17A level increased by 26.18 ng/L or less in the first seven days (log-rank test, P < 0.001). The 30-day survival rate was also significantly higher among patients whose baseline IL-17A level was lower than 210.25 ng/L (log-rank test, P = 0.011).
| Variables | Change from day 0 to day 7 | AUC (95% CI) | Sensitivity (%) | Specificity (%) | P |
|---|---|---|---|---|---|
| IL-17A | ≥ 26.18 ng/L increase | 0.634 (0.532 - 0.736) | 56.1 | 84.9 | 0.005 |
| Albumin | ≥ 5.5 g/L decrease | 0.651 (0.561 - 0.740) | 67.4 | 62.1 | 0.001 |
| D-dimer | ≥ 771 ng/mL increase | 0.622 (0.522 - 0.722) | 40.9 | 97.7 | 0.010 |
Abbreviations: CI, confidence interval; CRP, C-reactive protein; IL-17A, interleukin 17A.
Rates of mortality in the first 30 days according to the determined cut-off values for change in IL-17A from baseline to day seven and baseline IL-17A level (A, change of IL-17A from baseline at day7, red line: ≥ 26.18 ng/L increase, blue line: increase below 26.18 ng/L or decrease; B, Baseline IL-17A, red line: ≥ 210.25 ng/L, blue line: < 210.25 ng/L).
5. Discussion
In this study, we determined that the IL-17A level measured at hospital admission and its change on day 7 were prognostic mortality indicators in patients with COVID-19 (SARS-CoV-2 infection). Over the last two years, many people have lost their lives because of ARDS associated with severe COVID-19. Features of systemic hyperinflammation characterize severe COVID-19, referred to as macrophage activation syndrome (MAS) (10). Patients with SARS-CoV-2 infection have been shown to have high Th17 cell counts. These cells produce IL-17A, which in cases of severe disease can induce the production of proinflammatory cytokines such as IL-1, IL-6, and TNF-α, indicators of hyperinflammation (9, 11-13). We examined the prognostic value of IL-17A in this study because it plays a central role in tissue inflammation by inducing the release of proinflammatory and neutrophil-mobilizing cytokines and is easily assessed.
A meta-analysis evaluating the relationship between disease severity and IL-17A in COVID-19 showed that IL-17A levels were higher in patients than in controls regardless of disease severity, consistent with our findings. In that meta-analysis, IL-17A was even higher in moderate and severe diseases than in controls. However, as in our study, the increase in serum IL-17A level was significantly greater in patients with severe disease compared to those with moderate disease, but the relationship was not as strong as in other comparisons (8). Another meta-analysis observed no difference between severe and non-severe patients (14). Therefore, given the available data, IL-17A is not a strong enough indicator of disease severity.
In the literature, many routine biomarkers have been evaluated as indicators of poor prognosis associated with severe disease and mortality in COVID-19. Consistent with previous studies, we observed a significant difference in D-dimer, CRP, ferritin, albumin, and NRL values in non-surviving patients (9, 15, 16). IL-17A had comparable mortality predictive power to these biomarkers. IL-17A plays an essential role in tissue repair and remodeling (e.g., bone resorption, ventricular tissue after myocardial infarction) and noninfectious processes such as inflammatory and autoimmune disorders and cancer (5, 16, 17). Low specificity resulting from a high false positivity rate is also a potential factor reducing the predictive power of IL-17A for mortality.
In a small study from Italy (n = 31), there was no difference between IL-17A values at admission and on day 7. However, the study included patients with mild hypoxemia (18). In our study, serum IL-17A levels decreased significantly compared to baseline in surviving patients and increased significantly in non-surviving patients. Xu et al. also observed an increase in IL-17A from baseline to day 10 in a small group of non-surviving patients (n = 6), although the change was not significant (19). The use of serum IL-17A levels as a prognostic indicator of mortality and ARDS in COVID-19 patients has been proposed as a hypothesis (5, 20). The findings supported this hypothesis that IL-17A was an independent prognostic factor for the risk of COVID-19-related mortality in a study of intensive care patients by Rubina et al. (21). The higher survival rates among patients whose initial IL-17A level and 7-day change in IL-17A were below the determined cut-off values in the present study also support its use as a prognostic marker.
The main limitation of our study is that it was conducted in a single center and clinics treating patients with severe diseases. Therefore, the study does not reflect overall mortality and disease severity rates for COVID-19. Another limitation is the small sample size. In addition, the study did not include a detailed analysis of other diseases that may cause increased IL-17A levels or characteristics that may affect immune responses, such as age, sex, and race.
5.1. Conclusions
The results of this study suggest that IL-17A, an important part of the immune system, may have utility in predicting mortality in COVID-19 patients.