1. Background
In December 2019, a new coronavirus called SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) led to an outbreak in Wuhan, China. The World Health Organization (WHO) called it COVID-19 (coronavirus disease 2019) (1). COVID-19 is spread by droplets and has become a pandemic that causes a global crisis (2). According to the latest statistics of WHO, by 14 November 2022, the number of confirmed COVID-19 patients was 631 935 687 in the world, of whom 6 588 850 patients died (3). In Iran, COVID-19 infected 7 558 950 people and killed 144 612 patients by 14 November 2022 (4). Typical symptoms are fever, cough, fatigue, and gastrointestinal symptoms such as diarrhea. It might be seen with symptoms of pneumonia and respiratory distress syndrome (2). Treatments include supportive oxygenation therapy, antiviral therapy, steroids, nonsteroidal anti-inflammatory drugs, and immunosuppressive medications (2). COVID-19 symptoms are more common in patients with underlying diseases such as diabetes, hypertension, hyperlipidemia, and pulmonary complications, leading to a higher mortality rate in these patients (5).
One of the symptoms of COVID-19 is thrombotic disorders, which have a high prevalence, especially in patients admitted to the intensive care unit, leading to high mortality of COVID-19 patients (6-8). COVID-19 causes tissue inflammation, hypoxia, and intravascular coagulation, leading to venous and arterial thrombosis in patients (6, 9). These disorders may take the form of venous sinus thrombosis, carotid artery thrombosis, stroke pulmonary embolism, cardiac arrhythmia, acute coronary syndrome, arterial embolism in the limbs (9-11), and thrombosis in the portal vein, mesenteric artery, and the graft aortas (6-8, 10, 12-14). Deep vein thrombosis (DVT) refers to the formation of blood clots within large deep veins, commonly in the leg or pelvis (15). The most common and life-threatening consequence of DVT is pulmonary embolism. It occurs when a blood clot locates in the way of the bloodstream to the lungs (15).
Generally, DVT and pulmonary embolism could be grouped as venous thromboembolism (VTE) (16). Various studies have identified pulmonary embolism and vascular thrombosis as 2 important complications of COVID-19 and emphasized its early detection (7, 8). Wang et al. reviewed data from 1026 patients and showed that 11% of COVID-19 patients manifested thrombotic symptoms. They emphasized that COVID-19 patients that are more likely to have thrombosis should start antithrombotic prophylaxis immediately (14). Besides, in other studies, the pulmonary embolism and DVT frequencies ranged from 4 - 19% and 6 - 20% of COVID-19 patients, respectively (7, 8). Due to the prevalence, pathogenicity, and high mortality of thrombotic complications caused by COVID-19, identifying clinical symptoms is very important to prevent them (7, 8, 13, 14, 17).
2. Objectives
The present study aimed to investigate the relationship between clinical and laboratory findings with the occurrence of thrombotic events (such as DVT and pulmonary embolism) in COVID-19 patients.
3. Methods
3.1. Patients
All patients referred to Taleghani Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran, between February and September 2020, were included in the study. Inclusion criteria were a positive polymerase chain reaction (PCR) test for COVID-19, COVID-19 clinical symptoms, and COVID-19 typical lung involvement confirmed by a computed tomography (CT) scan. Exclusion criteria were a history of thrombophilia, pulmonary embolism, DVT, and being treated with anticoagulants at the time of admission. The patients first received a 200-mg loading dose of intravenous remdesivir (followed by 100 mg daily), a bi-daily 8-mg dose of intravenous dexamethasone, and a daily 40-mg dose of oral pantoprazole.
3.2. Clinical and Laboratory Parameters
The laboratory data, including the levels of lactate dehydrogenase (LDH), D-dimer, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and counts of lymphocyte and neutrophil, along with clinical findings such as oxygen saturation and lung involvement percentage, were retrospectively collected from patients’ clinical files. Then, the incidence of the following thrombotic events was evaluated: DVT was determined by a Doppler ultrasound scan, and pulmonary embolism was evaluated by CT angiography. The outcomes included thrombosis in the right or left main pulmonary artery, thrombosis in the right or left sub-segmental pulmonary artery, and thrombosis in the right or left deep vein. Clinical and laboratory findings of COVID-19 patients with thrombotic events were compared with COVID-19 patients without thrombotic events.
3.3. Statistical Analysis
The normality of data distribution was evaluated by the Kolmogorov-Smirnov goodness of fit test. A Student t test, Mann-Whitney U test, and Pierson and Spearman correlation tests were used to compare groups based on the normality of data distribution. A chi-squared test was employed to compare the categorical variables. Multiple logistic regression models were applied to examine the association of study variables with thromboembolic events. To this end, the dependent variable was defined as having a thromboembolic event or not having any thromboembolic events. Moreover, multiple logistic regression models were performed to evaluate the contribution of study variables to the prediction of mortality related to thromboembolic events in COVID-19 patients, where the dependent variable was defined as either death or being alive. All statistical analyses were analyzed using SPSS version 19 (IBM SPSS, Chicago, IL, USA) with a significance level of 0.05.
4. Results
4.1. Descriptive Data
A total of 114 patients (44% female and 56% male) with an average age of 59 years were included in the study. The minimum age was 18 years, and the maximum age was 90 years. Twenty-four patients developed thrombotic complications and were categorized into the thrombotic group. The rest 90 patients did not manifest thrombotic complications and were categorized into the non-thrombotic or control group. The mean age in both thrombotic and non-thrombotic groups was 59 years. In the control group, 39 people (43%) were women, and 51 people (57%) were men, while in the thrombotic group, 11 people (46%) were women, and 13 people (54%) were men. The descriptive data of patients are listed in Table 1.
| Variables | Non-thrombotic | Thrombotic | P Value |
|---|---|---|---|
| Age | 59.18 ± 14.2 | 59.46 ± 18.4 | 0.93 |
| Sex | 0.93 | ||
| Women | 39 (43.3) | 11 (45.8) | |
| Men | 51 (56.7) | 13 (54.2) | |
| Death | < 0.001 | ||
| Dead | 0 (0) | 5 (20.8) | |
| Alive | 90 (100) | 19 (79.2) | |
| Thrombotic events | |||
| RMPAT | 0 (0) | 3 (12.5) | |
| LMPAT | 0 (0) | 4 (16.7) | |
| RSPAT | 0 (0) | 8 (33.3) | |
| LSPAT | 0 (0) | 9 (37.5) | |
| RDVT | 0 (0) | 5 (20.8) | |
| LDVT | 0 (0) | 2 (8.3) |
Abbreviations: RMPAT, right main pulmonary artery thrombosis; LMPAT, left main pulmonary artery thrombosis; RSPAT, right sub-segmental pulmonary artery thrombosis; LSPAT, left sub-segmental pulmonary artery thrombosis; RDVT, right deep vein thrombosis; LDVT, left deep vein thrombosis.
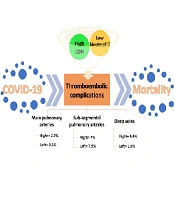
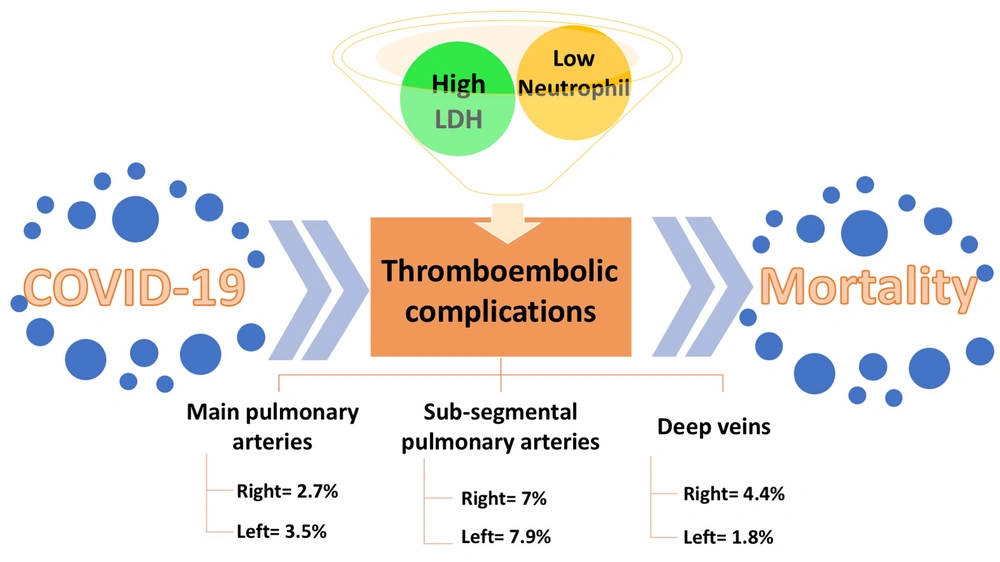
The prevalence of thrombosis in the right main pulmonary artery was 2.7% of all patients (12.5% of the thrombotic group), equal to 3 cases. The prevalence of thrombosis in the left main pulmonary artery was 3.5% of all patients (16.7% of the thrombotic group), equal to 4 cases. Eight cases (7% of all and 33.3% of thrombotic patients) showed thrombosis in the right sub-segmental pulmonary artery, while 9 cases (7.9% of all and 37.5% of thrombotic patients) manifested thrombosis in the left sub-segmental pulmonary artery. The prevalence of right DVT (RDVT) was 4.4% of all and 20.8% of thrombotic patients, equal to 5 cases, while the frequency of left DVT (LDVT) was 1.8% of all and 8.3% of thrombotic patients, equal to 2 cases (Table 1).
4.2. Intergroup Analyses
The total number of patients who died was 5, all of whom were in the thrombotic group. The mortality rate in the thrombotic group was 20%. The chi-square test showed a statistically significant relationship between being in the control and patient groups and death outcome (P < 0.001). According to the Kolmogorov-Smirnov normality test, only the age variable followed the normal distribution, which was compared between the 2 groups by an independent t test. The rest of the variables did not follow the normal distribution; hence, the Mann-Whitney U test was employed to compare them between the 2 groups.
The variables, including mean age, mean day of hospitalization, PaO2 saturation, levels of CRP and ESR, and lymphocyte count, showed no significant differences between the 2 groups (Table 2). However, as shown in Table 2, the mean level of LDH was 1004.3 ± 299.3 in the thrombotic group, which was significantly higher than that of the non-thrombotic group (777.1 ± 263; P < 0.001). The mean of neutrophil counts was 50.3 ± 39 and 81 ± 7.2 in the thrombotic and non-thrombotic groups, respectively, showing a significant decrease in the thrombotic group (P = 0.002). Besides, the chi-square test showed no statistically significant relationship between thrombocytopenia and the incidence of thrombotic events (P = 0.47).
| Variables | Non-thrombotic | Thrombotic | P Value |
|---|---|---|---|
| Days of hospitalization | 11.28 ± 6.3 | 14.63 ± 11 | 0.23 |
| Mean PaO2 saturation (%) | 83.95 ± 7.1 | 84.48 ± 8.8 | 0.46 |
| Mean CRP level | 16.74 ± 13.2 | 25.89 ± 30.5 | 0.39 |
| Mean ESR level | 38.36 ± 17.8 | 39.18 ± 19 | 0.8 |
| Mean LDH level | 777.19 ± 263 | 1004.33 ± 299.3 | < 0.001 |
| Mean lymphocyte count | 12.9 ± 6.3 | 12.1 ± 15.8 | 0.07 |
| Mean neutrophil count | 81.05 ± 7.2 | 50.33 ± 39 | 0.002 |
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase.
4.3. Multivariate Logistic Regression
The results of multivariate logistic regression analysis for predicting thromboembolic events based on the study variables are presented in Table 3. None of the studied variables made a significant contribution to predicting thromboembolic incidence in COVID-19 patients. Table 4 presents the multivariate logistic regression analysis of predictors of COVID-19-related death. No significant association was found between the study variables and death.
| Variables | P Value | Exp. (B) | 95% CI |
|---|---|---|---|
| Age | 0.54 | 0.98 | 0.94 - 1.03 |
| Sex | 0.60 | 0.63 | 0.11 - 3.51 |
| Days of hospitalization | 0.18 | 1.07 | 0.97 - 1.18 |
| Mean PaO2 saturation (%) | 0.55 | 0.99 | 0.99 - 1.01 |
| Mean CRP level | 0.23 | 1.02 | 0.98 - 1.05 |
| Mean ESR level | 0.82 | 0.99 | 0.95 - 1.04 |
| Mean LDH level | 0.37 | 1 | 0.99 - 1.01 |
| Mean lymphocyte count | 0.75 | 0.97 | 0.84 - 1.13 |
| Mean neutrophil count | 0.14 | 0.93 | 0.86 - 1.02 |
| Constant | 0.57 | 13.38 |
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; Exp., exponential.
| Variables | P Value | Exp. (B) | 95% CI |
|---|---|---|---|
| Age | 0.24 | 1.06 | 0.96 - 1.17 |
| Sex | 0.99 | 0.99 | 0.64 - 15.27 |
| Days of hospitalization | 0.62 | 0.95 | 0.79 - 1.14 |
| Mean PaO2 saturation (%) | 0.71 | 0.99 | 0.99 - 1.01 |
| Mean CRP level | 0.70 | 0.98 | 0.89 - 1.07 |
| Mean ESR level | 0.76 | 1.01 | 0.95 - 1.07 |
| Mean LDH level | 0.52 | 1 | 0.99 - 1.01 |
| Mean lymphocyte count | 0.57 | 1.26 | 0.56 - 2.83 |
| Mean neutrophil count | 0.38 | 1.37 | 0.67 - 2.78 |
| Constant | 0.29 | 0 |
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; Exp., exponential.
5. Discussion
COVID-19 causes tissue inflammation, hypoxia, and intravascular coagulation, leading to thromboembolic complications in patients (6, 12). The present study investigated the relationship between clinical and laboratory findings with thrombotic events, such as DVT and pulmonary embolism, in COVID-19 patients. Twenty-four out of 114 COVID-19 patients (21%) developed thrombotic complications. Nineteen patients (16.6%) experienced pulmonary thrombotic complications, and 5 patients (4.4%) had DVT. In a systematic review and meta-analysis of 42 studies, the pooled rate of pulmonary embolism was reported 13%, and DVT was reported in 20% of overall COVID-19 patients (7). However, in another systematic review and meta-analysis of 30 studies, pulmonary thromboembolism was reported as 4 - 19%, and DVT was between 6% and 14% (8). Galeano-Valle et al. (18) indicated that 24 (3%) out of 785 COVID-19 patients had VTE. None of the patients had a history of thrombophilia, pregnancy, or prolonged travel. Among the patients with venous thrombosis, 11 patients (45.8%) had only symptoms of pulmonary embolism, 9 (37.5%) had symptoms of DVT, and 4 (16.6%) had symptoms of both DVT and pulmonary embolism. Among 15 patients with pulmonary embolism, 6 (40%) had severe symptoms of pulmonary embolism, and 9 (60%) had moderate symptoms (18).
In the current study, the total number of patients who died was 5, all of whom were in the thrombotic group. The mortality rate was 20% in the thrombotic group. Moreover, a significant association was found between death and thromboembolic complications. Consistently, Malas et al. found that the pooled mortality rate was 23% in thromboembolism patients (7). They reported that the pooled odds of death were 74% higher in the thromboembolic COVID-19 patients compared to the control COVID-19 patients (7). Klok et al. examined the symptoms of pulmonary embolism, DVT, myocardial infarction (MI), and arterial embolism in patients admitted to intensive care units. The sample consisted of 184 patients, of whom 41 (22%) died and 78 (43%) were discharged. The mean hospital stay was 14 - 17 days, and all patients received prophylaxis of vascular thrombosis. The results showed that prophylaxis treatment effectively reduced mortality (19).
The variables, including mean age, mean day of hospitalization, PaO2 saturation, levels of CRP and ESR, and lymphocyte count, showed no significant differences between the thrombotic and non-thrombotic groups. Parallel to this finding, Lee et al. also reported no significant difference in oxygen saturation between thrombotic and non-thrombotic COVID-19 patients (20). Riyahi et al. conducted a multicenter study on 413 COVID-19 patients and found pulmonary embolism in 25% of hospitalized patients (11). They found no significant differences between the level of CRP and ESR, as well as lymphocyte and platelet counts between patients with pulmonary embolism and those without pulmonary embolism (11). Besides, they found no significant differences in the hospitalization length between the embolic and non-embolic groups, which parallels the finding of the present study (11).
Nevertheless, Ierardi et al. found that CRP was independently associated with the presence of DVT (21). However, based on their report, every 1-unit rise in the CRP level could only 0.9% increase the risk of DVT (21). Another study on 1026 patients with 113 thrombotic patients reported that patients with high CRP were more likely to have thrombosis (14). In a cohort study by Lee et al., the CRP level was suggested as an indicator of VTE in COVID-19 patients. They also reported that hospitalization length, lymphocyte count, and platelet count were associated with VTE in COVID-19 patients (20). The contradictions between the studies might be due to the different sample sizes. It might be better to pool the results of studies in large meta-analyses to reach a robust conclusion. In the current study, the mean level of LDH was 1004.3 ± 299.3 in the thrombotic group, which was significantly higher than that of the non-thrombotic group (777.1 ± 263). Accordingly, LDH is reported to be associated with pulmonary embolism in COVID-19 patients (11).
A systematic review and meta-analysis of 12 articles with combined 1083 patients showed that COVID-19 patients with thrombotic complications had higher LDH levels than those without thrombotic events; in other words, they showed LDH as a risk factor for thrombosis in COVID-19 patients (Figure 1) (22). High levels of LDH are closely related to the severity, poor prognosis, and higher mortality of COVID-19 (23, 24). Besides, LDH has been previously reported as a predictor for pump-induced thrombosis in those with continuous-flow left ventricular assist devices (25). Nevertheless, the logistic regression model showed that neither LDH nor any other evaluated factors significantly contributed to predicting thrombotic events and mortality. Regarding the small sample size, further analyses on larger sample sizes are required to determine the predictors of thrombosis in COVID-19. Interestingly, based on logistic and linear regression models in a multicenter large cohort study on 3531 patients, CRP and LDH were unable to predict thromboembolism in COVID-19, which is in accordance with the present study (20).
The role of lactate dehydrogenase and neutrophil levels in thromboembolic COVID-19 patients. Thromboembolic complications are common consequences of COVID-19, involving pulmonary arteries and deep veins. Lactate dehydrogenase and neutrophil levels are significantly higher and lower in thromboembolic COVID-19 patients than in those without thromboembolic manifestations. Thus, they can be used as biomarkers for early diagnosis and even prediction of thromboembolic complications to decrease the mortality rate.
The mean of neutrophil counts was 50.3 ± 39 and 81 ± 7.2 in the thrombotic and non-thrombotic groups, respectively, showing a significant decrease in the thrombotic group (Figure 1). The roles of neutrophils in innate immune system responses have been extensively investigated (26, 27). It has been found that neutrophils are key players in thromboembolism in COVID-19 patients (27). One of the mechanisms that neutrophils use to trap and remove SARS-CoV-2 is the formation of neutrophil extracellular traps (NETs) in a process called NETosis (26). In this process, which is a unique kind of cell death, neutrophils release their decondensed chromatin and granular enzymes to the extracellular space to trap and destroy pathogens. An excess NET formation is strongly associated with acute respiratory distress syndrome and thrombosis (27). Interestingly, NETs are abundantly found in severely damaged COVID-19 lung tissue and are associated with micro-thrombosis of the alveolar capillaries (27). Regarding neutrophil death during NETosis, neutropenia in COVID-19 patients with thrombotic complications might be justifiable. However, neutrophilia is a hallmark of severe COVID-19 patients regardless of thromboembolic complications (5).
Besides, the current study showed no statistically significant relationship between thrombocytopenia and the incidence of thrombotic events. The small sample size might affect the statistical significance of the results. In a parallel study, it was reported that the platelet count between the thrombotic and non-thrombotic COVID-19 patients was not significant (11). This finding might indicate that neutrophils have superior roles compared to platelets in the formation of thromboembolism in COVID-19 patients. However, several studies have reported a relationship between thrombocytopenia and thrombotic events in severe COVID-19 patients (5, 28, 29). Severe COVID-19 patients have elevated platelet consumption, leading to mild thrombocytopenia in 60% - 95% of severe cases (1, 30, 31). The surprising fact that such critically ill COVID-19 patients with systemic inflammation and coagulopathy have only mild thrombocytopenia showed that the compensatory platelet production rate increased accordingly (28).
In a consensus statement published in July 2022, several biomarkers were considered for the prognosis and diagnosis of thrombotic complications of COVID-19. High levels of CRP, D-dimer, calprotectin, P-selectin, and urinary 11-dehydrothromboxane B2, along with thrombocytopenia and the presence of NETosis, are suggested as thrombotic biomarkers of COVID-19 (32).
The current study had limitations. The main limitation is the small sample size, which could affect the power of the study. Therefore, further research is recommended to develop and confirm the initial findings reported in this paper. Another limitation is the absence of patients with other thrombotic complications, such as myocardial infarction, transient ischemic attack, and other systemic embolisms, due to the limited sample size. The other important limitation is the fact that patients who were taking anticoagulants were not included in the analysis. Yet, future research is needed to compare the incidence of events in patients receiving anticoagulant therapy with those not receiving this treatment.
5.1. Conclusions
The present study showed that thromboembolic complications are considerable consequences of COVID-19, associated with a high risk of mortality. Hence, the early diagnosis and even prediction of thromboembolic complications could facilitate the treatment and decrease the mortality rate. In this regard, this study shows that LDH and neutrophil levels in thromboembolic COVID-19 patients are significantly higher and lower than those without thromboembolic manifestations. Such biomarkers could serve in the prognosis and management of thromboembolism in COVID-19 patients.