1. Background
Urinary tract infections (UTIs) are among the most common infections which diagnosed in both hospitalized cases and community setting (1-3). The prevalence of extended spectrum beta-lactamases (ESBL) producing uropathogenic bacteria has increased in the recent years (4-6). These microorganisms derived from urinary tract that commonly cause sepsis, respiratory tract, and intra-abdominal infection (7).
Extended-spectrum of beta-lactamases are enzymes that able to inactivate beta-lactam antibiotics such as penicillins, cephalosporins, and monobactams by hydrolysis. Extended-Spectrum of Beta-Lactamases can be transferred basically on plasmids, hydrolyze third generation cephalosporins which are inhibited by clavulanic acid, tazobactam or sulbactam (7). The ESBL enzymes are predominantly found in Escherichia coli and Klebsiella spp. however, may also be found in other species of Enterobacteriaceae (4, 5, 8). The treatment of infections caused by ESBL-producing bacteria is often complicated by the concomitant resistance to other classes of antibiotics such as fluoroquinolones, aminoglycosides and trimethoprim-sulfamethoxazole (9).
2. Objectives
This study aimed to investigate the ESBL prevalence of E. coli and Klebsiella spp. which isolated from urine samples from in and outpatients with their resistance profiles during nine years period; in order to provide the microbiological point of view in anti-infective management of urinary tract infections.
3. Methods
Our data was collected from the Haydarpasa Numune training and research hospital (HNEAH), which has a 725 bed capacity, in the period between January 2004 and December 2012, retrospectively. The patients were demonstrated clinical symptoms compatible with urinary tract infection from the outpatient clinics and inpatients after 48 hours of hospitalization. Ethical approval was granted by Haydarpasa Numune training and research hospital ethical committee (HNEAH-KAEK/09).
The mid-stream urine specimens were transported to the Microbiology laboratory, and were then inoculated in 5% sheep blood agar (Oxoid, United Kingdom) and MacConkey agar (Oxoid, United Kingdom) by a calibrated loop of 10 μL using the streak plate method immediately. The growth of the ≥ 105 colony forming units per milliliter in the culture was included in our study. The duplicate isolates from the same patient were excluded.
Overall, 13975 clinical isolates were analyzed and identified by standard culture, biochemical characteristics and differentiated to species level using BBL Enteric/Nonfermenter ID Kit (Becton Dickinson, USA) and Vitek2 system (bioMerieux, France). Antibiotic susceptibility testing was performed by the Kirby-Bauer disk diffusion method and microbroth dilution assay using Vitek2 system (bioMerieux, France) according to clinical and laboratory standards institute (CLSI) criteria (10). The following antibiotics (Antimicrobial Susceptibility Disks, OxoidTM, United Kingdom) were tested: amoxicillin-clavulanic acid, trimethoprim-sulfamethoxazole, gentamicin, amikacin, cefoperazone-sulbactam, ciprofloxacin, levofloxacin, ertapenem, imipenem, meropenem and nitrofurantoin. The isolates were classified as susceptible, intermediate or resistant, according to the breakpoints established by the CLSI (10). The ESBL producers were detected by CLSI double disk diffusion method of three different beta-lactam antibiotics (ceftazidim 30 μg, cefotaxime 30 μg and cefuroxime 30 μg) with and without clavulanic acid (11). The quality control was performed by testing Escherichia coli ATCC 25922 and Klebsiella pneumoniae ATCC 700603.
3.1. Statistical Methods
The statistical analysis was performed with hypothesis test in the NCSS (Number cruncher statistical system) 2007&PASS (power analysis and sample size) 2008 Statistical Software (Utah, USA) program. In addition, in order to evaluate the study, data with definitive statistical methods (average, standard deviation, median, frequency, and ratio), for the comparison of the qualitative data Pearson Chi Square test, the Yates continuity correction test, the Fisher’s Exact test, and for the trend analysis, the Chi-Square test linear-by-linear test were used. The Pearson’s correlation analysis was used for the correlation between the ESBL proportions in years. The results were considered in 95% confidence intervals, and statistically significant with P values < 0.05.
4. Results
A total of 212,521 urine samples of patients were analyzed and 27,901 of them were positive for growth. The overall study included 12,897 E. coli and 1078 Klebsiella spp. isolates that 9,299 (72.1%) E. coli and 629 (58.3%) Klebsiella spp were isolated from female patients. 2461 E. coli (19%) and 365 (33.8%) Klebsiella spp. were isolated from inpatients. 1715 (13.3%) E. coli and 202 (18.7%) Klebsiella spp isolates were ESBL producers.
4.1. Escherichia coli
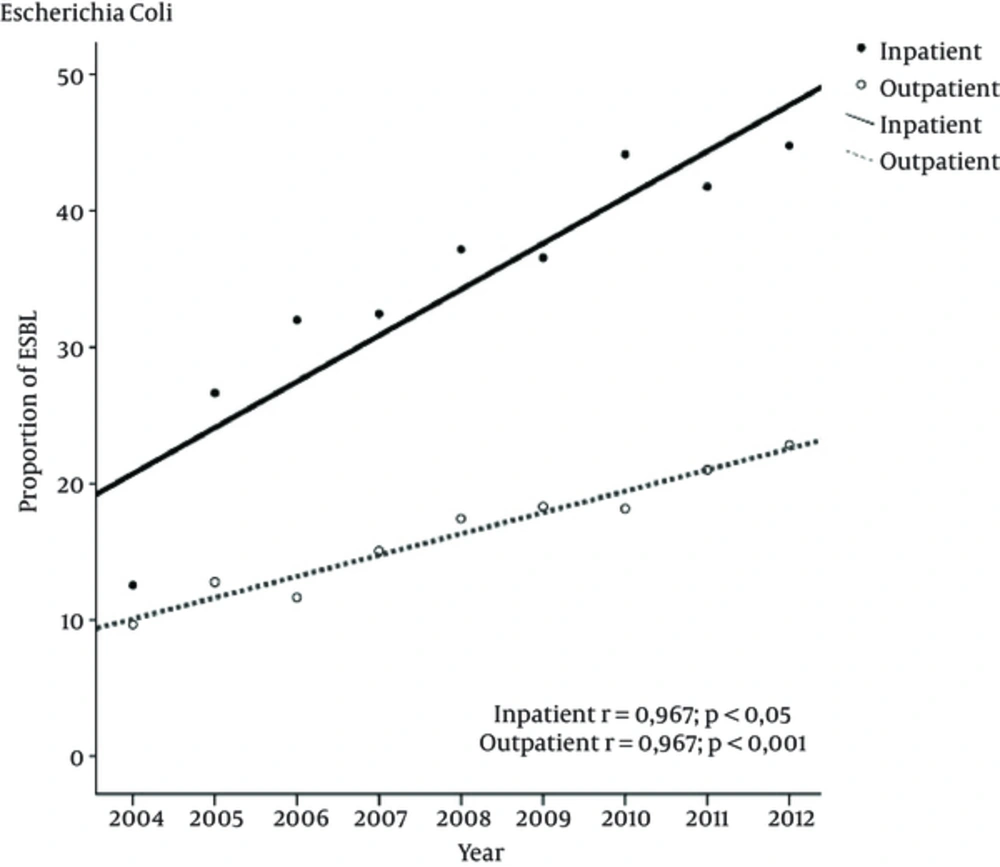
Between 2004 and 2012, the prevalence of ESBL producing E. coli increased significantly from 12.5% to 44.7% (P < 0.001) for inpatients and from 9.6% to 22.8% (P < 0.001) for outpatients. This time trend was linear and has an increasing rate in the ESBL producing isolates which were observed in all patient groups (Figure 1). Of the 2641 inpatients, 1906 (72.1%) with 7393 of the outpatients 5,323 (72%) being female. When 2004 was compared to 2012 for ESBL prevalence, there was a significant increase in both sexes (P < 0.001) (Table 1).
P, significance for correlation; r, Pearson’s correlation index. Means and standard error of the means of ESBL producing rates from 2004 to 2012. The linear regression showed significant increase over time for E. coli both in-and outpatients (P < 0.001) for both, correlation was linear (P < 0.001) for both.
| Escherichia coli | Klebsiella spp | |||||
|---|---|---|---|---|---|---|
| 2004 | 2012 | P Valueb | 2004 | 2012 | P Valueb | |
| All | 99/947 (10.5) | 400/1803 (22.2) | 0.001c | 13/78 (16.7) | 74/188 (39.4) | 0.001c |
| In-outpatient | ||||||
| Inpatients | 33/263 (12.5) | 180/402 (44.8) | 0.001c | 7/28 (25.0) | 46/76 (60.5) | 0.003c,d |
| Outpatients | 66/684 (9.6) | 320/1401 (22.8) | 0. 001c | 6/50 (12.0) | 28/112 (25.0) | 0.095d |
| Sex | ||||||
| Female | 68/631 (10.8) | 468/1414 (33.1) | 0.001c | 3/24 (12.5) | 37/118 (31.4) | 0.105d |
| Male | 31/316 (9.8) | 132/389 (33.9) | 0.001c | 10/54 (18.5) | 37/70 (52.9) | 0.001c |
| Sex-inpatient | ||||||
| Inpatients-Female | 27/193 (14.0) | 129/293 (44.0) | 0.001c | 3/10 (30.0) | 24/42 (57.1) | 0.167e |
| Inpatients-Male | 6/70 (8.6) | 51/109 (46.8) | 0.001c | 4/18 (22.2) | 22/34 (64.7) | 0.009c,d |
| Sex-outpatient | ||||||
| Outpatients-Female | 41/438 (9.4) | 239/1121 (21.3) | 0.001c | 0/14 (0.0) | 13/76 (17.1) | 0.207e |
| Outpatients-Male | 25/246 (10.2) | 81/280 (28.9) | 0.001c | 6/36 (16.7) | 15/36 (41.7) | 0.038d |
aValues are expressed as No. (%).
bPearson ki kare test.
cP < 0.001.
dYates Continuity Correction.
eFisher’s Exact test.
The resistance to the first line choice nitrofurantoin for the uncomplicated UTI was as low as 3% for the non-ESBL producer and 10% for the producing E. coli isolates in 2012, regardless of being an in or outpatient. The resistance rates of amoxicillin-clavulanic acid increased significantly in inpatients; especially for the ESBL producers. The susceptibility of the non-ESBL producer isolates fluoroquinolones and trimethoprim-sulfamethoxazole was significantly increased. The highest susceptibility detected for amikacin and there was no resistance to carbapenems. For all the antibiotics tested, the ESBL-producing E. coli isolates showed lower susceptibility rates compared to the non-producers (P < 0.05). There was no significant resistance difference as far as ESBL-producer isolates were concerned in both in-and outpatients. The resistance trends over these years are presented with details in Table 2.
| Antibiotic | Patient | ESBL | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | P Value | Trend |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amoxicillin-Clavulonic acid | Inpatients | [-] | 27 | 23,9 | 22,8 | 25 | 27 | 24,3 | 29,4 | 33,2 | 32 | *a | ↑ |
| [+] | 63,6 | 60,3 | 68,4 | 64,4 | 73,8 | 69,4 | 80,5 | 89,4 | 93,3 | **b | ↑ | ||
| Outpatients | [-] | 22,3 | 14,5 | 19,8 | 19,9 | 21,2 | 19,7 | 22,1 | 23,3 | 27,9 | ** | ↑ | |
| [+] | 47 | 50,4 | 46,5 | 45 | 47,4 | 39,4 | 37,8 | 44,9 | 50,6 | ↑ | |||
| Cefoperazone-Sulbactam | Inpatients | [+] | 27,3 | 28,8 | 27,4 | 13,7 | 15,5 | 14,9 | 15 | 18,9 | 26,7 | ↓ | |
| Outpatients | [+] | 16,7 | 16,8 | 18,9 | 10,6 | 9,6 | 6,9 | 5,2 | 6,6 | 13,1 | ** | ↓ | |
| Amikacin | Inpatients | [-] | 3,5 | 3,5 | 4,5 | 3,3 | 3,4 | 2,4 | 2,1 | 2,2 | 1,8 | ↓ | |
| [+] | 3 | 4,1 | 3,2 | 4,1 | 6,8 | 8,3 | 5,3 | 6,1 | 7,2 | ↑ | |||
| Outpatients | [-] | 0,8 | 3,6 | 3,2 | 2 | 2,7 | 1,3 | 1 | 1 | 0,9 | ** | ↑ | |
| [+] | 3 | 3,5 | 3,9 | 3,8 | 3,8 | 4,5 | 4,3 | 4,4 | 5,9 | ↑ | |||
| Gentamicin | Inpatients | [-] | 22,6 | 10,9 | 15,3 | 19,1 | 18,4 | 17,1 | 14,7 | 13,6 | 12,2 | * | ↓ |
| [+] | 51,5 | 37 | 42,1 | 50,7 | 42,7 | 38 | 52,2 | 56,1 | 49,4 | * | ↓ | ||
| Outpatients | [-] | 11 | 14,1 | 18,4 | 17,9 | 17,2 | 16,6 | 16,7 | 14 | 12,1 | ↑ | ||
| [+] | 40,9 | 42,5 | 39,4 | 40 | 38,3 | 38,2 | 41,2 | 36,9 | 39,4 | ↓ | |||
| Ciprofloxacin | Inpatients | [-] | 44,3 | 43,8 | 45 | 47,4 | 44,8 | 38,1 | 35 | 33,7 | 31,1 | ** | ↓ |
| [+] | 72,7 | 75,3 | 83,2 | 83,6 | 81,6 | 71,9 | 69,9 | 75 | 72,8 | ↑ | |||
| Outpatients | [-] | 32 | 36,6 | 36,8 | 33,1 | 34,9 | 32,6 | 31,5 | 29,1 | 20,5 | ** | ↓ | |
| [+] | 75,8 | 72,6 | 72,4 | 80 | 77,5 | 67,5 | 66,1 | 68,2 | 70 | * | ↓ | ||
| Levofloxacin | Inpatients | [-] | 40,4 | 44,3 | 45,5 | 46,7 | 43,7 | 35,2 | 34,3 | 31 | 29,7 | ** | ↓ |
| [+] | 66,7 | 74 | 85,3 | 84,9 | 78,6 | 69,4 | 65,5 | 77,3 | 71,7 | ↑ | |||
| Outpatients | [-] | 35,6 | 37,4 | 36,5 | 32,4 | 35 | 33,8 | 31 | 28,9 | 19,8 | ** | ↓ | |
| [+] | 72,7 | 69,9 | 73,2 | 70 | 76,1 | 65,4 | 64,4 | 66,1 | 68,4 | ↓ | |||
| Nitrofurantoin | Inpatients | [-] | 13 | 11,4 | 5,9 | 4,6 | 6,9 | 4,3 | 4,2 | 3,8 | 2,3 | ** | ↓ |
| [+] | 6,1 | 12,3 | 17,9 | 17,8 | 14,6 | 15,7 | 12,4 | 11,4 | 10,6 | ↑ | |||
| Outpatients | [-] | 9,9 | 9,3 | 7,7 | 5,3 | 5,8 | 5,6 | 4 | 3,6 | 2,2 | ** | ↓ | |
| [+] | 19,7 | 13,3 | 9,4 | 11,3 | 11,5 | 13,8 | 12,4 | 9,9 | 9,7 | ↓ | |||
| Trimethoprim-Sulfamethoxazole | Inpatients | [-] | 52,2 | 50,7 | 52,5 | 46,7 | 52,3 | 45,2 | 41,3 | 42,9 | 32,4 | ** | ↓ |
| [+] | 45,5 | 52,1 | 78,9 | 67,1 | 73,8 | 65,3 | 72,6 | 74,2 | 68,9 | * | ↑ | ||
| Outpatients | [-] | 59,9 | 47 | 47,1 | 45 | 42,8 | 45,2 | 40,2 | 40,7 | 27,8 | ** | ↓ | |
| [+] | 66,7 | 54,9 | 59,1 | 67,5 | 61,2 | 62,6 | 56,7 | 54 | 62,5 | ↓ |
aP < 0.05.
bP < 0.01.
4.2. Klebsiella spp.
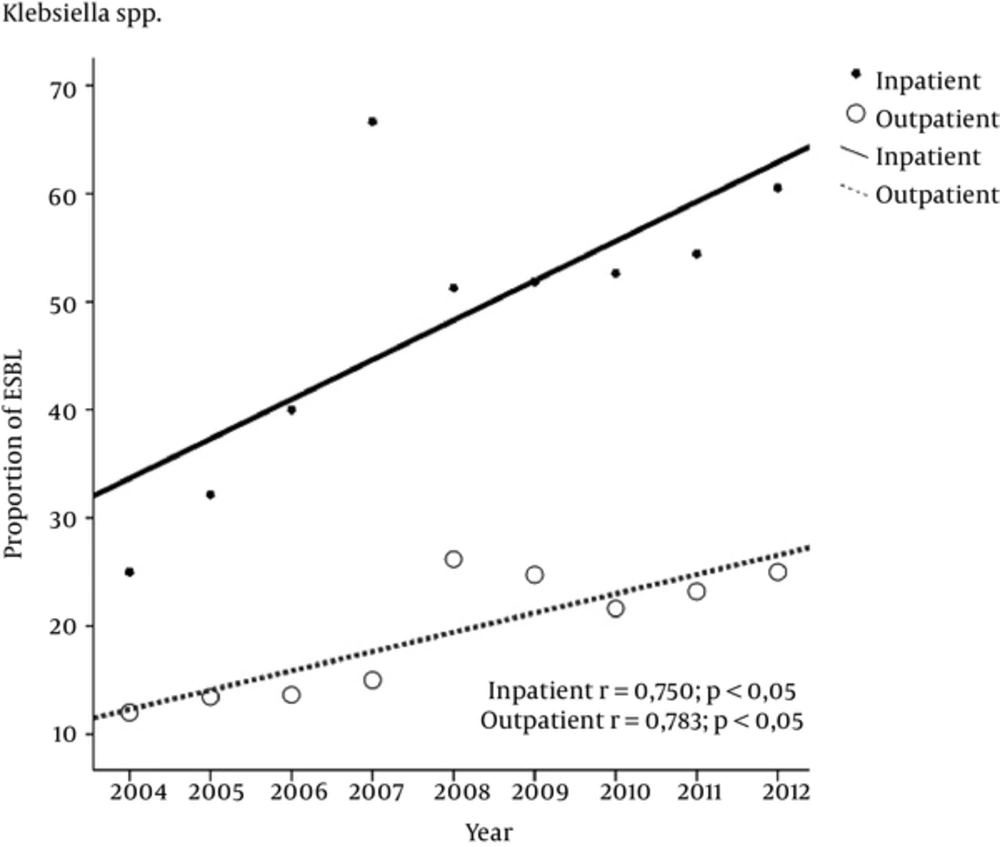
During 2004 to 2012, the prevalence of ESBL producing Klebsiella spp. increased from 25% to 60.5% (P < 0.003) for inpatients and from 12% to 25% (P < 0.095) for outpatients (Table 1). The increase was significant and linear in both groups (P < 0.005) (Figure 2). 195 inpatients (53.4%) and 434 outpatients (60.8%) were female. The ESBL prevalence significantly increased in the male inpatients in particular (P < 0.001) from 2004 to 2012.
The resistance rate of the amoxicillin-clavulanic acid and cefoperazone-sulbactam to ESBL producing Klebsiella spp. increased in all patients. For inpatients, the susceptibility rates of fluoroquinolones in the ESBL producers decreased, whereas fluctuations were observed in other groups. There was no significant difference in the susceptibility rates for gentamicin, amikacin, and nitrofurantoin among the groups. Interestingly, the trimethoprim-sulfamethoxazole resistance significantly decreased for non-ESBL producers in outpatients, whereas it’s significantly increased in other groups. In general, the ESBL producing isolates had increased resistance rates in all antibiotics when compared to the non ESBL producers. The ertapenem resistance among the ESBL-producing Klebsiella spp. isolates emerged in 2008 and 2009 in inpatients and outpatients, respectively. The carbepenem resistance reached to 7% in the outpatients and 15% in inpatients (Table 3).
| Antibiotic | Patient | ESBL | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | P Value | Trend |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amoxicillin-Clavulonic acid | Inpatients | [-] | 23,8 | 21,1 | 40 | 50 | 47,4 | 38,5 | 40,7 | 33,3 | 40 | ↑ | |
| [+] | 42,9 | 66,7 | 60 | 75 | 90 | 85,7 | 80 | 81,4 | 95,7 | **a | ↑ | ||
| Outpatients | [-] | 20,5 | 17,2 | 21,1 | 26,5 | 38,7 | 41,4 | 36,8 | 40,7 | 40,5 | ** | ↑ | |
| [+] | 50 | 44,4 | 66,7 | 66,7 | 77,3 | 69,6 | 75 | 76,9 | 89,3 | ** | ↑ | ||
| Cefoperazone-Sulbactam | Inpatients | [+] | 14,3 | 33,3 | 30 | 25 | 40 | 35,7 | 53,3 | 51,2 | 54,3 | ** | ↑ |
| [+] | 16,7 | 22,2 | 33,3 | 50 | 36,4 | 47,8 | 41,7 | 42,3 | 50 | ↑ | |||
| Gentamicin | Outpatients | [-] | 19 | 10,5 | 20 | 50 | 31,6 | 30,8 | 22,2 | 22,2 | 20 | ↑ | |
| [+] | 28,6 | 33,3 | 30 | 50 | 20 | 28,6 | 36,7 | 37,2 | 39,1 | ↑ | |||
| Inpatients | [-] | 18,2 | 12,1 | 15,8 | 14,7 | 12,9 | 11,4 | 17,2 | 19,8 | 20,2 | ↑ | ||
| [+] | 33,3 | 22,2 | 33,3 | 33,3 | 36,4 | 30,4 | 33,3 | 38,5 | 35,7 | ↑ | |||
| Amikacin | Outpatients | [-] | 9,5 | 10,5 | 6,7 | 0 | 15,8 | 7,7 | 7,4 | 8,3 | 6,7 | ↓ | |
| [+] | 14,3 | 22,2 | 20 | 25 | 25 | 21,4 | 13,3 | 14 | 10,9 | ↓ | |||
| Inpatients | [-] | 4,5 | 5,2 | 2,6 | 2,9 | 6,5 | 4,3 | 3,4 | 5,8 | 6 | ↑ | ||
| [+] | 16,7 | 22,2 | 16,7 | 16,7 | 13,6 | 8,7 | 8,3 | 11,5 | 7,1 | ↓ | |||
| Levofloxacin | Inpatients | [-] | 23,8 | 26,3 | 33,3 | 50 | 42,1 | 46,2 | 48,1 | 38,9 | 36,7 | ↑ | |
| [+] | 42,9 | 44,4 | 50 | 75 | 60 | 50 | 60 | 76,7 | 71,7 | *b | ↑ | ||
| Outpatients | [-] | 29,5 | 24,1 | 23,7 | 23,5 | 29 | 21,4 | 20,7 | 24,4 | 36,9 | ↑ | ||
| [+] | 66,7 | 55,6 | 66,7 | 66,7 | 63,6 | 43,5 | 45,8 | 61,5 | 60,7 | ↓ | |||
| Ciprofloxacin | Inpatients | [-] | 28,6 | 21,1 | 33,3 | 50 | 47,4 | 53,8 | 51,9 | 38,9 | 40 | ↑ | |
| [+] | 57,1 | 55,6 | 50 | 75 | 65 | 57,1 | 60 | 76,7 | 69,6 | ↑ | |||
| Outpatients | [-] | 31,8 | 25,9 | 26,3 | 26,5 | 30,6 | 20 | 19,5 | 25,6 | 35,7 | ↑ | ||
| [+] | 66,7 | 55,6 | 66,7 | 66,7 | 59,1 | 47,8 | 50 | 61,5 | 64,3 | ↓ | |||
| Nitrofurantoin | Inpatients | [-] | 33,3 | 31,6 | 46,7 | 50 | 52,6 | 46,2 | 33,3 | 44,4 | 43,3 | ↑ | |
| [+] | 42,9 | 33,3 | 50 | 50 | 60 | 57,1 | 50 | 65,1 | 78,3 | ** | ↑ | ||
| Outpatients | [-] | 43,2 | 34,5 | 31,6 | 47,1 | 48,4 | 40 | 47,1 | 46,5 | 42,9 | ↓ | ||
| [+] | 83,3 | 55,6 | 66,7 | 50 | 45,5 | 47,8 | 58,3 | 57,7 | 75 | ↓ | |||
| Trimethoprim-Sulfamethoxazole | Inpatients | [-] | 23,8 | 26,3 | 33,3 | 50 | 47,4 | 38,5 | 29,6 | 22,2 | 16,7 | ↓ | |
| [+] | 57,1 | 44,4 | 60 | 75 | 60 | 71,4 | 80 | 74,4 | 71,7 | * | ↑ | ||
| Outpatients | [-] | 47,7 | 41,4 | 36,8 | 35,3 | 43,5 | 30 | 25,3 | 22,1 | 23,8 | ** | ↓ | |
| [+] | 66,7 | 55,6 | 83,3 | 66,7 | 54,5 | 60,9 | 79,2 | 73,1 | 71,4 | ↑ | |||
| Ertapenem | Inpatients | [+] | 0 | 0 | 0 | 0 | 20 | 21,4 | 13,3 | 18,6 | 15,2 | ↓ | |
| Outpatients | [+] | 0 | 0 | 0 | 0 | 0 | 13 | 8,3 | 19,2 | 10,7 | ↓ | ||
| Imipenem | Inpatients | [+] | 0 | 0 | 0 | 0 | 0 | 14,3 | 10 | 14 | 15,2 | ↑ | |
| Outpatients | [+] | 0 | 0 | 0 | 0 | 0 | 0 | 8,3 | 3,8 | 7,1 | ↓ | ||
| Meropenem | Inpatients | [+] | 0 | 0 | 0 | 0 | 0 | 14,3 | 10 | 14 | 13 | ↓ | |
| Outpatients | [+] | 0 | 0 | 0 | 0 | 0 | 0 | 8,3 | 3,8 | 7,1 | ↓ |
aP < 0.01.
bP < 0.05.
5. Discussion
This is a nine-year surveillance study in order to evaluate the antibiotic resistance patterns and ESBL prevalence of E. coli and Klebsiella spp. which is recovered from UTI among in and outpatients in a tertiary care hospital. Routinely, the data that collected in targeted clinics on antibiotic resistance, is remarkable for developing guidelines, which been used in our hospital since 2003.
The ESBL production among Enterobacteriaceae is a worldwide concern. The rates have been reported in a variety of studies as 1.7% to 19% (8, 11-14). In our study, the ESBL production rates were 13.3% and 18.7% for E. coli and Klebsiella spp., respectively. The studies reporting ESBL production rates in our country showed similar results (15, 16). Despite the common increasing in ESBL production in both of in and outpatients, this rates can vary among both regions and hospitals. The data on the prevalence of ESBL isolates among outpatients are rarely reported (17-19).
5.1. Escherichia coli
Extended-spectrum of beta-lactamases producing E. coli has become the most troublesome causative agent of UTIs. In 2012, the overall prevalence in our study among inpatients and outpatients was 44.7% and 22.8%, respectively. The lower rates were reported in outpatients from Turkey (16, 17, 20). Although in Netherlands, the prevalence of ESBL-producing E. coli in outpatients is lower than our country, there was a significant increasing from 2004 (0.1%) to 2009 (1%) (18). However, China (21) and Tanzania (22) have higher rates when compared to our study. The prevalence of ESBL-producing E. coli for inpatients in Switzerland is 6.6% (23), 10.4% in Saudi Arabia (8) and 52.9% in China (21). ESBL producing E. coli incidences was significantly higher in females (73.6%) than males (26.4%) at the end of study.
The resistance to different antibiotics in ESBL producing E. coli is frequent and at least in part, due to the fact that genes coding is located on the same plasmids (6). In both inpatients and outpatients, roughly 70% of ESBL producing E. coli were resistant to both trimethoprim-sulfamethoxazole and fluoroquinolones. Several studies have been shown fluoroquinolones and trimethoprim-sulfamethoxazole to be highly active against E. coli (24-28) in contrast to dramatically decreasing susceptibility rates among ESBL-producers (20, 21, 25, 29). Fluoroquinolone resistance was reported 85% in ESBL producing E. coli isolates in a study in Nepal in 2012 (29) and in 2010 Italian study reported this rate to be even higher (85% - 90%) in ESBL-producing E. coli isolates (30). The trimethoprim sulfamethoxazole and fluoroquinolones susceptibility rates in non-ESBL-producing E. coli were between 20% and 30% in recent studies. These drugs are not useful for empirical treatment however, if the specific ESBL-producing microorganism is finally susceptible, these antibiotics could be used. The nitrofurantoin susceptibility rates for both non-ESBL and ESBL-producing E. coli were 98% and 90%, which were the same as those that observed in similar studies (21, 29, 31). A study conducted in Brazil reported a higher resistance rate of 35% for nitrofurantoin in ESBL-producing E. coli (19). Also a review article from Turkey showed similar susceptibility rates, just as in our study for E. coli (20), suggesting the use of nitrofurantoin for the first-line empirical oral treatment of community-acquired uncomplicated UTIs.
The resistance rate of amoxicillin-clavulanic acid was 90% in inpatients and 50% in outpatients respectively, for ESBL-producing E. coli; therefore it may not be a good choice in our region for UTIs. Some other studies showed similar results but some found higher susceptibility rates in ESBL-producing E. coli isolates (18, 21, 29).
Amikacin was found to have a high activity against all E. coli, however gentamicin had higher resistance rates in ESBL-producing E. coli as shown in other studies (13, 29, 30). Our findings showed that all E. coli exhibited a 100% susceptibility to carbapenems, therefore they were the most effective choice of drug against ESBL producing E. coli. However, it is a concern for spreading of carbapenemase producers which will increase the incidence of MDR bacteria.
5.2. Klebsiella spp.
The prevalence rates of ESBL producing Klebsiella spp. are increasing worldwide. Our rates in 2012, with an increasing trend, reached to 60.5% and 25% for both inpatients and outpatients, respectively. Similar results were reported in a previous study (14). An approximation frequency of 24% was observed in Turkey (16), 41.5% in Tanzania (22) and 16.5% in Nepal (29). The rates of ESBL producing Klebsiella spp. increased in male inpatients in particular, in our study.
The resistance to antibiotic classes was even higher than in E. coli. The ESBL-producing Klebsiella spp. resistance rates in all patients in 2012 were above 60% for all of the antibiotics tested, except for cefaperazone-sulbactam, aminoglycosides, and carbapenems. The resistance rates were reasonably high and maximally resistant (95.7%) to amoxicillin-clavulanic acid. A high resistance of 78.3%, 71.7%, and approximately 70%, were shown in nitrofurantoine, trimethoprim-sulfamethoxazole and fluoroquinolones, respectively. A moderate resistance of 50.4% was shown towards cefoperazone-sulbactam and 39.1% to gentamicin. The amikacin (10.9% resistance) was the most effective antibiotic. A study in Tanzania also showed similar results (22). In other studies which conducted in Italy (30), Brazil (14), and Nepal (29), the resistance rates were lower compared to our results.
The carbapenem resistance was alarming against the ESBL-producers. It’s increased from 0% to 15% for the inpatients. Some of studies that conducted in other countries have not reported such high resistance for carbapenems in UTIs (25, 26). Recently, carbapenems have been increasingly used as an empirical treatment for complicated UTIs and these results affect in promoting the selection of drug-resistant bacteria and an increasing prevalence of fungal infections and flora imbalance.
Non-ESBL producing Klebsiella spp. had a remarkable increasing resistance for nitrofurantoine compared to E. coli. On the contrary, the trimethoprim-sulfamethoxazole resistance was decreased from 47.7% to 23.8% due to the limited using. Therefore, it can be a preferred choice in UTIs. Similar results were reported from Netherlands (26). However, a study carried out in Tanzania, reported a high rate for the trimethoprim-sulfamethoxazole and a lower rate for nitrofurantoine (22).
The major limitation in our study was that we could not classify complicated or uncomplicated UTIs due to the lack of information on the database. Moreover, the molecular characterization of the ESBL isolates could not be studied. This is a study that was conducted for long-term as it needed nine years in order to evaluate the antibiotic resistance of ESBL-producing and non-producing E. coli and Klebsiella spp. isolates which were recovered from both in and outpatients in both of sexes.
In conclusion, our study demonstrated a significant increase in prevalence of ESBL producing E. coli and Klebsiella spp. during 2004 to 2012 for UTIs. The ESBL prevalence of E. coli significantly increased in both sexes and also in-outpatients, however for the Klebsiella spp., it’s significantly increased just in male inpatients. The resistance rates of antibiotics, especially fluoroquinolones, showed a significant increase for ESBL producing E. coli and Klebsiella spp. The carbapenem resistance was alarming against the ESBL producing Klebsiella spp. The nitrofurantoin may be the first line choice for the uncomplicated UTI caused by E. coli. The trimethoprim-sulfamethoxazole may be a good choice for treatment of non-ESBL E. coli and Klebsiella spp. Regular surveillance studies need to be performed in order to select adequate empirical antibiotic regimens and controlling the antibiotic resistance, especially monitoring of ESBLs.