1. Background
In the last 20 years, there has been a nearly tenfold increase in hospital-acquired fungal infections, with 80% caused by Candida species (1). Although there have been significant advances in the diagnosis and antifungal treatments of infectious diseases, one-third of patients with Candida infection die (2). More than half of the invasive Candida infections are candidemia, which accounts for 4.5% of bloodstream infections (2). Causative agents of candidemia have changed over time (3). There have been increasing reports of high mortality in non-albicans Candida species, including C. glabrata, C. krusei, and C. tropicalis (4). Fluconazole and echinocandins are the most widely used antifungal therapies worldwide (5, 6). Due to the increase of isolates with intrinsic and acquired resistance to antifungal agents, detecting candidemia agents, their antifungal susceptibility, and risk factors will reduce hospital mortality (7). It is essential to choose an empirical treatment and modify it according to the microbiology laboratory results.
2. Objectives
We aimed to epidemiologically examine the cases of candidemia in a tertiary hospital to determine the risk factors associated with mortality, the species causing candidemia, and their antifungal susceptibility.
3. Methods
3.1. Patients and Fungal Isolates
Adult patients with healthcare-acquired candidemia hospitalized at the University of Health Sciences, Prof. Dr. Cemil Taşcıoğlu City Hospital, between June 2016 and October 2020, were included in the study. The demographic and clinical data of the patients (age, gender, presence of malignancy, concomitant disease, antibiotic use, presence of febrile neutropenia, antifungal use, history of operation, duration of total parenteral nutrition, and mortality status) were collected from medical records and the hospital automation system retrospectively. In addition, the microbiology laboratory results of the patients (Candida species growing in the blood culture, antifungal sensitivity, and simultaneous bacterial and fungal growth in cultures) were also evaluated. Patients diagnosed with Coronavirus disease 2019 (COVID-19) were excluded from the study. Blood cultures were incubated in an automated blood culture system (BACTEC FX, BD, USA) at the Central Microbiology Laboratory. The germ tube test and MALDI-TOF MS (Vitek MS, bioMérieux, France) were used to identify Candida species.
3.2. Definition
Candidemia was defined as at least one positive blood culture for Candida species in patients with fever or other clinical signs of infection. When the blood culture was positive for bacteria in addition to the Candida species, the case was defined as having concurrent bacteremia. In patients with recurrent candidaemia, we counted only the first case; however, it was accepted as a new case if the isolation of a Candida strain occurred after 30 days.
3.3. Antifungal Susceptibility Testing
Antifungal susceptibility results were obtained using the Sensititre YeastONE® (SYO) Antifungal Susceptibility Panel (Thermo Fisher Scientific, UK) based on the colorimetric method. The antifungal susceptibility results were evaluated according to Clinical and Laboratory Standards Institute (CLSI) M27-S4 and M60 documents (8). Two reference strains, C. krusei ATCC 6258 and C. parapsilosis complex ATCC 22019, were included in each test as quality control isolates.
3.4. Statistical Analysis
Descriptive statistics were presented as frequency, percentage, mean, standard deviation (SD), median (median), minimum (min.), and maximum (max.). Fisher's exact or Pearson χ2 test was used to analyze the relationships between categorical variables. The Shapiro-Wilk test determined the normality assumption before exploring the difference between the two groups. The Mann-Whitney U test was used when it did not follow the normal distribution. Analyses were performed using IBM SPSS Statistics for Windows version 21.0 (Statistical Package for the Social Sciences, IBM Corp., Armonk, NY, USA). Statistically, P values less than 0.05 were considered significant.
4. Results
A total of 217 patients had yeast growth in their blood cultures. Among these patients, 51 patients with confirmed COVID-19 diagnosis and 11 patients whose data could not be accessed were excluded. A total of 155 patients were included in the study.
4.1. Candida Strains and Incidence of Candidemia
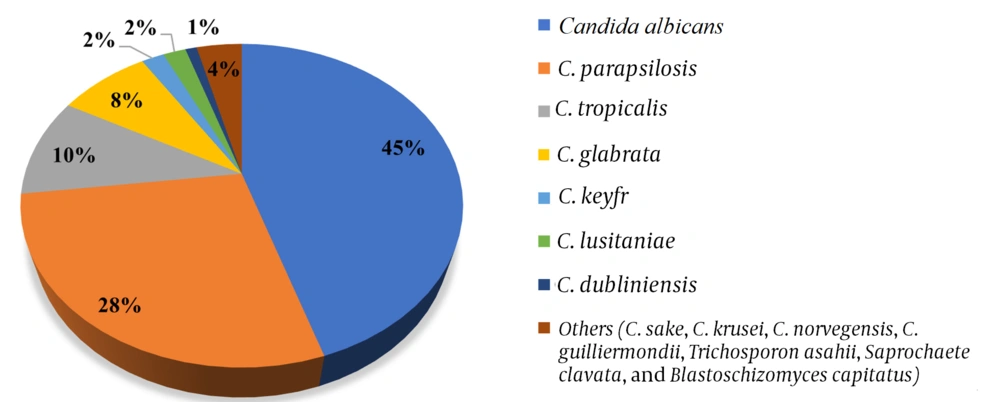
Candida albicans in 69 (45%) patients and non-albicans yeast species in 86 (55%) patients were isolated in the blood culture. The isolated non-albicans yeast species in order of frequency were C. parapsilosis complex (43, 28%), C. tropicalis (16, 10%), C. glabrata (12, 8%), C. keyfr (3, 2%), C. lusitaniae (3, 2 %), C. dubliniensis (2, 1%), and other species (7, 4%), including C. sake (n = 1), C. krusei (n = 1), C. norvegensis (n = 1), C. guilliermondii (n = 1), Trichosporon asahii (n = 1), Saprochaete clavata (n = 1), and Blastoschizomyces capitatus (n = 1) (Figure 1). A total of 7050, 14210, 16558, 17286, and 9862 blood cultures were sent to the microbiology laboratory in July - December 2016, 2017, 2018, 2019, and 2020, respectively, and 316, 563, 535, 611, 1368 were reported as positive. The incidences of candidemia were 0.92, 0.72, 0.99, 0.97, and 2.28 per 1,000 cases in July-December 2016, 2017, 2018, 2019, and 2020, respectively.
4.2. Antifungal Susceptibility Patterns of Isolates
Among C. albicans isolates, 67 were sensitive to anidulafungin, micafungin, caspofungin, and voriconazole, and 62 were sensitive to fluconazole. Two C. albicans strains were resistant to fluconazole, and one strain was resistant to voriconazole. Also, 13 C. parapsilosis strains were resistant to fluconazole, and 11 were intermediate (I) + susceptible-dose-dependent (SDD) to voriconazole. All C. tropicalis isolates were sensitive to anidulafungin, micafungin, and caspofungin, while one strain showed resistance to voriconazole and fluconazole, and nine strains were I+SDD against voriconazole. All 12 C. glabrata strains were susceptible to anidulafungin and micafungin, while all were I + SDD against fluconazole (Tables 1 and 2). The susceptibility rates for anidulafungin, micafungin, caspofungin, voriconazole, and fluconazole were as follows: 97, 97, 97, 97, and 90% in C. albicans, 95, 95, 98, 72, and 67% in C. parapsilosis complex, and 100, 100, 100, 38, and 63% in C. tropicalis. The susceptibility rates on the basis of species as a percentage for anidulafungin, micafungin, and caspofungin in C. glabrata were 100, 100, and 92%, respectively.
| Yeast Species | Anidulafungin | Micafungin | Caspofungin | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | I + SDD | R | U | S | I + SDD | R | U | S | I + SDD | R | U | ||
| Candida albicans | 67 | 2 | 0 | 0 | 67 | 1 | 1 | 0 | 67 | 2 | 0 | 0 | 69 |
| C. parapsilosis complex | 41 | 1 | 1 | 0 | 41 | 1 | 1 | 0 | 42 | 0 | 1 | 0 | 43 |
| C. tropicalis | 16 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 16 |
| C. glabrata | 12 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 11 | 1 | 0 | 0 | 12 |
Antifungal Susceptibility of the Most Common Candida Species for Echinocandins
| Yeast Species | Voriconazole | Fluconazole | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S | I + SDD | R | U | S | I + SDD | R | U | ||
| Candida albicans | 67 | 1 | 1 | 0 | 62 | 5 | 2 | 0 | 69 |
| C. parapsilosis complex | 31 | 11 | 0 | 0 | 29 | 1 | 13 | 0 | 43 |
| C. tropicalis | 6 | 9 | 1 | 0 | 10 | 5 | 1 | 0 | 16 |
| C. glabrata | 0 | 0 | 0 | 12 | 0 | 12 | 0 | 0 | 12 |
Antifungal Susceptibility of the Most Common Candida Species for Azoles
4.3. Clinical Data
The age of the patients ranged from 20 to 90; 90 (58%) were male, and 65 (42%) were female. Of the patients, 88 (57%) were hospitalized in the intensive care unit, 19 (12%) in internal medicine, 17 (11%) in hematology, 16 (10%) in general surgery, five (3%) in infectious diseases wards, and others (7%) in clinics including neurology, oncology, urology, obstetrics and gynecology, cardiac surgery, and radiation oncology. Seventy patients died (Table 3). There was no significant difference in mortality and survival between C. albicans and non-albicans species and other species in patients with candidemia. The crude mortality rate within 30 days after the initial positive culture was 45% (n = 70) (Table 3). Among the study patients, 83 (88%) had malignancies. The most common cancer types seen in candidemia patients were hematological cancers (Hodgkin lymphoma, non-Hodgkin lymphoma, acute lymphocytic leukemia, acute lymphoblastic leukemia, B-cell lymphoma, chronic myeloid leukemia, multiple myeloma) (20/83, 24%), colon cancer (14/83, 17%), brain tumor (14/83, 17%), and stomach cancer (12/83, 14%). Ovarian cancer (n = 3), esophageal cancer (n = 3), rectal cancer (n = 3), lung cancer (n = 3), bladder cancer (n = 3), and endometrial cancer (n = 2) were also detected. Other underlying diseases were hypertension (60, 38%), chronic kidney failure (19, 12%), chronic obstructive pulmonary disease (18, 11.6%), diabetes (12, 13%), heart failure (10, 6%), and febrile neutropenia (7, 5%) (Table 3).
| Characteristics | Values |
|---|---|
| Age (mean ± standard deviation) | 62.36 ± 16.8 |
| Gender | |
| Male | 90 (58) |
| Female | 65 (42) |
| Mortality | 70 (45) |
| Underlying diseases | |
| Malignancy | 83 (88) |
| Hypertension | 60 (38) |
| Diabetes | 12 (13) |
| Chronic kidney failure | 19 (12) |
| Chronic obstructive pulmonary disease | 18 (11.6) |
| Heart failure | 10 (6) |
| Febrile neutropenia | 7 (5) |
| Simultaneously culture positivity | |
| Healthcare-acquired bacterial infection | 65 (42) |
| Candiduria | 28 (18) |
| Pre-candidemia treatments | |
| Antibiotic use | 155 (100) |
| Empirical antifungal therapy | 141 (91) |
| Total parenteral nutrition | 100 (65) |
| Invasive interventions before candidemia | |
| Central venous catheter | 119 (77) |
| Mechanical ventilation | 90 (58) |
| Gastrointestinal surgery | 59 (38) |
Demographic and Clinical Data of Patients with Candidemia a
Simultaneously, bacteria were isolated in 65 (42%) bacterial cultures. The most frequently isolated bacterial species were Klebsiella pneumoniae (n = 21), Enterococcus species (n = 10), Acinetobacter baumannii (n = 22), Escherichia coli (n = 10), followed by Serratia marcescens, Proteus mirabilis, methicillin-resistant Staphylococcus aureus, and methicillin-resistant coagulase-negative staphylococci. Twenty (95%, 20/21) of the K. pneumoniae isolates and six (27%, 6/22) of the A. baumannii isolates were resistant to meropenem, all E. coli isolates (100%, 10/10) were ESBL (extended spectrum beta lactamase)-positive, and one Enterococcus faecium was resistant to vancomycin. In 37 (24%, 37/155) patients, Candida species simultaneously grew in urine (n = 27), catheter tips (n = 8), urine + catheter tip (n = 1), trachea (n = 2), and peritoneal fluid samples (Table 1). One hundred (65%) patients received TPN, and 119 (77%) were catheterized. All patients (100%) had a history of broad-spectrum antibiotic use (Table 3). A total of 141 (91%) patients received empirical antifungal therapy, 61 (43%) micafungin, 36 (26%) caspofungin, 28 (20%) anidulafungin, 13 (9%) fluconazole, and three (2%) voriconazole (Table 3).
Considering the invasive interventions before candidemia, a central venous catheter was applied to 119 (77%) patients and mechanical ventilation to 90 (58%) patients. A total of 59 (38%) patients had a history of gastrointestinal surgery. In patients with gastrointestinal surgery, the most common isolated yeasts were C. albicans (n = 27), C. parapsilosis complex (n = 18), C. glabrata (n = 6), C. tropicalis (n = 5), C. dubliniensis (n = 1), C. guilliermondii (n = 1), and C. norvegensis (n = 1) (Table 3).
There was no significant difference between C. albicans and non-albicans Candida and other yeast species in patients with candidemia in terms of underlying diseases, candiduria, total parenteral nutrition, antifungal use, antibiotic use, central venous catheter use, mechanical ventilation use, and gastrointestinal surgery.
5. Discussion
Detecting causative agents of bloodstream infections is vital for antifungal therapy and surveillance. In a multicenter study in Japan among 289 cases, C. albicans was the most frequent causative agent (44.3%), followed by C. parapsilosis complex (25.3%), C. glabrata (15.9%), and C. tropicalis (4.8%). The survival rate was higher for patients with C. parapsilosis complex candidemia than those with other species or mixed fungemia in the Kaplan-Meier analysis (2). In a study in Turkey with 102 adult candidemia patients, 36.3% of patients had C. albicans, and 63.7% had non-albicans, including C. parapsilosis complex (22.5%), C. tropicalis (16.7%), and C. glabrata (12.7%) (1). Candida albicans was the most prevalent species, followed by C. parapsilosis among candidemia patients, according to many reports in the literature (6, 9). On the contrary, C. parapsilosis has been reported as the most commonly isolated Candida strain in other studies (10, 11).
In our study of hospital-acquired candidemia patients, C. albicans accounted for 45% of all cases, and the most common yeast species other than C. albicans were C. parapsilosis complex (28%), C. tropicalis (10%), and C. glabrata (8%). These data indicated that C. albicans was a significant cause of candidemia, but infections due to non-albicans species were also problematic. Further, we did not find a significant difference in risk factors and mortality between yeast species in the current study. In some epidemiologic studies, mortality was higher in patients with non-albicans Candida infections than in those with C. albicans infections (12). However, no difference or higher mortality rates with C. albicans have also been reported in many studies (13).
Continuous surveillance plays a crucial role in controlling infections due to intrinsic antifungal-resistant species such as C. glabrata, which has a decreased susceptibility to azoles and resistance to amphotericin B. Candida glabrata candidemia is more common among the elderly and cancer patients. The ability of C. parapsilosis complex to colonize human skin is a commonly reported cause of catheter-related infections (11, 12). Our study indicated that bloodstream infections due to C. albicans were common. Still, it is essential to know the increasing proportion of C. glabrata, C. parapsilosis complex, and C. tropicalis.
In our study, the incidences of candidemia were 0.92, 0.72, 0.99, 0.97, and 2.28 per 1,000 cases in four study years (2016 - 2020). There was a notable increase in its incidence in 2020. Some studies reported up to a 5-10-fold increase in the incidence of candidemia during the COVID-19 pandemic (14, 15). Despite excluding patients with COVID-19, we observed a higher incidence than in previous years. Inappropriate antibiotic use and changes in the hospital environment during this era may have led to this observation. Additionally, 57% of the patients were hospitalized in the intensive care unit. The incidence rate reported from Turkey in the literature ranges from 0.56 to 11.5 per 1000 admissions (1, 10, 16, 17). The literature data indicated that ICU patients had higher candidemia risk and mortality than general ward patients (4). We suggested safe and effective prophylactic strategies, high-risk patient identification, and daily bathing with chlorhexidine to decrease the incidence (18).
In a study in Turkey, echinocandin sensitivity was more than 95%, C. parapsilosis complex had 8.7% fluconazole and 4.4% voriconazole resistance, and C. tropicalis had 5.9% fluconazole and 5.9% voriconazole resistance. Cross-resistance was also detected in two C. parapsilosis complex strains and one C. tropicalis strain against fluconazole (1). In another study by Dogan et al., 13% of C. parapsilosis strains showed resistance to fluconazole, and all species were susceptible to echinocandins (6). In our study, the susceptibility rate of echinocandins was more than 95% against Candida isolates. I + SDD and resistance rates for voriconazole and fluconazole were 3% and 10% in C. albicans, 25% and 33% in C. parapsilosis complex, and 56% and 38% in C. tropicalis. All 12 C. glabrata strains included in the study were SDD for flucanazole and uninterpretable for voriconazole. Our study and literature data indicated that echinocandins had good activity for Candida species, and voriconazole resistance rates were increasing in C. tropicalis and C. parapsilosis complex strains.
The broth microdilution method has been accepted as a standardized reference for antifungal susceptibility detection in Candida species. The SYO susceptibility system is a micro broth method that provides qualitative and quantitative minimal inhibitory concentration (MIC) values. In a study by Altınbaş et al., the antifungal susceptibility of 129 Candida isolates was evaluated by both SYO and CLSI M27-A3 BMD methods. The SYO method demonstrated an excellent performance for all antifungals except voriconazole and fluconazole. The authors agree that the SYO method is an effective and efficient alternative to the CLSI reference method (19). Philips et al. compared the two colorimetric broth microdilution antifungal susceptibility tests, SYO and MICRONAUT-AM, with 100 clinical Candida isolates.
Essential agreement of ≥ 90% was shown only for fluconazole, 5-flucytosine, caspofungin, and amphotericin B. SYO MICs were higher than MICRONAUT MICs for all antifungals, except for itraconazole. Only amphotericin B, fluconazole, and micafungin had a categorical agreement of ≥ 90%. The proportions of susceptibility for amphotericin B, fluconazole, and micafungin were comparable. The proportion of sensitive and I + SDD Candida strains for voriconazole (71.2% vs. 90.9%) and posaconazole (67.5% vs. 90.9%) was higher when using the MICRONAUT system. In comparison, it was higher for itraconazole (95.8% vs. 77.8%) and anidulafungin (93.9% vs. 72.7%) when Sensititre was used (SYO vs. MICRONAUT, respectively) (20).
In a study by Dalyan Cilo and Ener, the antifungal susceptibility of various Candida species was compared between the VITEK 2 automated system and the reference CLSI M27 microdilution method. They detected antifungal susceptibilities to amphotericin B, voriconazole, and fluconazole in 140 Candida strains and anidulafungin in 92 strains. The VITEK 2 MIC values at 24 hours for azole antifungals were one-fold higher than the CLSI MICs. Between the two methods, the essential agreement was > 90% for voriconazole and amphotericin B, while it was 85% for fluconazole. Amphotericin B showed the best (99.3%) categorical agreement, and the least categorical agreement was detected with voriconazole (85.7%).
VITEK 2 failed to detect resistance in one C. glabrata strain, which was found resistant to fluconazole by the reference method. Although the error rate was not very high, VITEK 2 could not detect one fluconazole-resistant C. parapsilosis complex or C. glabrata strain in this study (21). Our study showed that the SYO susceptibility system had promising activity in obtaining the results for anidulafungin, micafungin, caspofungin, voriconazole, and fluconazole susceptibilities in C. albicans, C. tropicalis, and C. parapsilosis complex. However, uninterpretable results were common in C. glabrata and other non-albicans yeast species. Additionally, the SYO susceptibility system was not good for evaluating amphotericin B susceptibility. Further studies are warranted to validate the fast antifungal susceptibility detecting systems.
Empiric antifungal therapy is crucial in managing candidemia, and delay in the initiation of treatment is an independent risk factor for high mortality. Previously, it has been reported that only 11 - 32% of patients with candidemia were treated with appropriate antifungal agents, and poor response to initial antifungal therapy was a risk factor for high mortality. It is also recommended to draw follow-up blood cultures to confirm the clearance of candidemia (22, 23). In a study by Kato et al., 68.5% of patients were treated empirically with echinocandins, and 16.3% received empiric fluconazole. They found the protective role of empiric fluconazole treatment against patient mortality (2). However, the choice of empirical antifungal therapy remains controversial.
Infectious Diseases Society of America guideline recommends echinocandins over fluconazole, particularly for those with moderate to severe illness (24, 25). In our study, 91% of patients received empiric antifungal therapy. Also, 88% of these patients received echinocandins, 9% fluconazole, and 2% voriconazole. According to the updated guidelines, there has been a shift in the usage of antifungals from azoles to echinocandins (26). Our findings are also consistent with this change, with echinocandins being the first choice in our hospital. The choice of antifungal treatment should also be based on local epidemiological data and the resistance profiles of microorganisms.
In a Turkish study, the crude candidemia mortality rate was 79.3% (1). In a multicenter study from Japan, of 289 patients, 27.7% died within 30 days of candidemia onset (2). Also, mortality rates of 18 - 66% have been highlighted in previous reports (1, 26, 27). In our study, the mortality rate was 45%. Different mortality rates in studies may be due to some confounding factors, such as the heterogeneity of study populations and the choice of different treatment protocols, including empirical therapy. Our study showed that despite the high rate of empiric antifungal use, mortality was still high in candidemia patients. This highlights the importance of timely and appropriate intervention. In addition, echinocandins should be prioritized in critically ill patients, given the higher resistance rates to azoles.
In a Turkish study, the most common risk factors associated with candidemia were broad-spectrum antibiotic use (98%), the presence of a urinary catheter (96.1%), the presence of a concomitant hospital-acquired infection (92.2%), and the use of CVC (80.4%) (1). In a different study, the most common risk factors were broad-spectrum antibiotic use (95.6%), CVC (97.8%), mechanical ventilation (64.4%), and urinary catheterization (73.3%) (28). In a study in Japan, independent risk factors for 30-day mortality were antibiotic use, advanced age (≥ 65 years), and a SOFA score ≥ 6 in patients with candidemia (2). In our study, the most common risk factors for candidemia were antibiotic use (100%), malignancy (88%), central venous catheter use (77%), TPN (65%), mechanical ventilation (58%), hypertension (38%), and history of gastrointestinal surgery (38%).
There was no significant difference in risk factors between C. albicans and non-albicans Candida and other yeast species in patients with candidemia (2). Although early studies reported that removing CVC is associated with an improved prognosis (29), the incidence of CVC removal did not differ between the mortality group and the surviving patients in other studies (2, 30). If there is no sign of infection at the CVC site, some researchers suggest starting antifungal treatment, observing the response, and not removing the CVC unless the patient worsens (31). We recommend that patients receiving TPN and/or undergoing gastrointestinal surgery be monitored closely for candidemia.
Previous studies reported concurrent bacteremia rates of 7 - 61.1% (32-34). In our study, 42% of the patients had concurrent bacteremia. Gram-negative bacteria were isolated from most patients; 95% of the K. pneumoniae isolates and 27% of the A. baumannii isolates were resistant to meropenem, and all E. coli isolates were ESBL-positive. The study indicated that extended-spectrum antibiotic use was also a significant risk factor for candidemia, and carbapenem resistance was problematic in Gram-negative bacteria, especially K. pneumoniae. Antibiotic stewardship programs, active surveillance of antimicrobial resistance, and infection control measurements are needed to prevent the emergence of resistant strains (35).
5.1. Conclusions
Incidences of candidemia and susceptibility patterns of strains may vary over time and amongst the regions. Candida albicans was the predominant strain, and echinocandins demonstrated the highest susceptibility rates against the most common strains isolated from the current study patients. It is of utmost importance to perform antifungal susceptibility tests to guide patient treatment.