1. Background
A major problem for livestock farmers is enterotoxaemia and foot and mouth disease, which cause many economic losses and hinder their exports. Vaccines contain adjuvants made from aluminum gel and oil. To prevent foot and mouth disease (FMD) and enterotoxemia, we developed a combined bivalent vaccine based on aluminum gel or oil adjuvant. Clostridium perfringens is an anaerobic, Gram-positive bacterium that produces spores. A healthy sheep or goat's digestive tract normally contains these bacteria, normally found in the soil and the microflora (1, 2). There are 18 types of Clostridium toxins, divided into five groups (alpha, beta, epsilon, and iota) (3). Enterotoxemia is caused by the liberation of exotoxins of C. perfringens in the intestines of sheep, goats, cattle, foals, and piglets. This bacterium causes a common intestinal disease.
Clostridium perfringens type A causes various diseases in humans and animals and is primarily pathogenic due to its exotoxins. Everyone is susceptible to C. perfringens type A food poisoning. Infant ruminants are usually affected by C. perfringens type B, which causes hemorrhagic mucosal enteritis and death. Babies may die within hours or 1 - 4 days (4, 5). The findings show that C. perfringens type C infects more animal species than other Clostridium species. Fatalities have been reported in pigs, cattle, sheep, horses, and humans. The presence of beta toxins and attachment of bacteria to genome villi are necessary for pathogenicity (6-8). In the case of C. perfringens type D, toxins are produced in the gut after the animal overeats a high-carbohydrate diet; they enter the bloodstream, affecting the kidneys and nerves. This disease is called "pulpy kidney" or overeating. The toxins affect the central nervous system and often lead to the animal's death (5, 9). Clostridium perfringens type (E) is the cause of enteritis with bleeding in calves.
Foot and mouth disease virus (FMDV) belongs to the family Picornaviridae and the genus Aphthovirus. It has seven serotypes (A, C, O, Asia, SAT 1, 2, 3) and numerous subspecies within each serotype (10). The virus's genome is single-stranded plus-sense RNA with an approximate size of 8.5 kb. The disease is characterized by fever and vesicles in the mouth, breast, and feet. Fever, pain, and discomfort severely affect livestock growth and economic unit productivity. Although it generally recovers in adult animals, it usually causes high mortality in young animals (11, 12). Today, inactivated virus vaccines are used worldwide. Currently, the most common type of FMD vaccine is the inactive type. This type of vaccine activates humoral immune responses instead of cellular immune responses by inducing neutralizing antibodies and activating CD4 cells.
Aluminum adjuvant vaccines can stimulate humoral immunity via IL-4 cytokine, B lymphocyte stimulation, and immunoglobulin secretion, mainly IgG (13). However, the vaccines are ineffective at long-term protection. Therefore, it is necessary to either repeat the vaccination or increase the length of the immunity period by changing the type of adjuvant so that a cheap and effective vaccine can be made that will create a favorable immune response against this viral and bacterial disease. The combined vaccines reduce the number of injections required by reducing the vaccine doses and increasing immunity against multiple infections. Consequently, interactions between combined immunogens can pose significant problems for the development of multivalent vaccines (7, 14, 15). Because multiple pathogens are present in animals, combined or simultaneous vaccination is appropriate for epidemic diseases, providing the long-term immune response to both antigens, and reducing the stress of vaccination for the animals.
2. Objectives
This study examined the immunogenic effects of combined vaccines for viral and bacterial diseases that included C. perfringens species (B_C_D) along with different adjuvants to make a more informed assessment of using combined vaccines. The FMD and Hemorrhagic Septicemia (HS) vaccine provides long-term immunity against foot and mouth disease and HS. Therefore, the present study aimed to develop a combined FMD enterotoxemia vaccine using an inactivated FMD vaccine and enterotoxemia toxoid.
3. Methods
3.1. Enterotoxin Production
Clostridium perfringens types B, C, and D were obtained from Razi Serum Institute (RVSRI)'s Anaerobic Department. The toxin production for types B, C, and D contained: Type B (CN 228), Type C (CN 301), and Type D (CN 409). After using peptone, Na2HPO4, NaCl salt, cysteine hydrochloride, yeast extract, vitamin solution, and essential elements of glucose 50% or final concentration of 1% and 50% dextrin solution after the sterile stage, the strain was inoculated and kept at a constant temperature (37°C) for six hours in a fermenter at a pH 7 - 7.5 according to the British Pharmacopoeia (16). According to the standard SOP, the production of toxins was confirmed by calculating the Minimum Lethal Dose (MLD) at BALB/c mice (weight 20 ± 2 g, 6 - 8 weeks).
3.2. Preparation of Toxin
In the continuation of the culture of each microorganism, one liter of each culture medium (B_C_D) was centrifuged separately (g = 8,000 for 30 minutes). Then, each supernatant was removed, and 60% ammonium sulfate was added to the proteins of the supernatant (AM04011000, Scharlau, Spain) and stirred at 4°C for one hour to form a precipitate. The sediments were collected with centrifugation (g = 8,000 for 30 minutes). Sediments were collected with Tris-HCl buffer (pH = 6.5). The next step was to dialyze the precipitates at 4°C with the same buffer (MWCO 10kDa). They were loaded on the column of size exclusion chromatography (gel filtration with G50) (Figure 1 and 2). The column output was collected, and then the optical absorbance of the fractions separated from the column was measured at 280 nm. To obtain toxins with higher purity, maximum optical absorption was selected and combined. In the end, it was done (MLD) again at BALB/c mice (weight 20 ± 2 g, 6 - 8 weeks) (2, 6).
Polyacrylamide gel electrophoresis (SDS-PAGE) on some fractions after column chromatography with Sephadex G-50 gel. Lane L: The protein size marker; Lane 1: The crude culture supernatant containing epsilon toxin (ETX) before chromatography; Lanes 2-8: Some fractions after chromatography that show the relative purified (Etx)-(Beta). Epsilon toxin weighing about 32.9 kDa.
Polyacrylamide gel electrophoresis (SDS-PAGE) on some fractions after column chromatography with Sephadex G-50 gel. Lane L: The protein size marker; Lane 1: The crude culture supernatant containing Beta toxin before chromatography; Lanes 2-8: Some fractions after chromatography that show the relative purified (Etx)-(Beta). Beta toxin weighing about (35-37) Da.
3.3. Preparation of Toxoid
Formaldehyde was added to the prepared toxins and kept for six days at 37°C until it became a toxoid (16).
3.4. Preparation of Antigen
The virus (O/2016/IR) was propagated on monolayer cell culture and BHK21 suspension. It was incubated for 24 hours at a temperature of 37°C and an atmosphere of 5% CO2 (17, 18). Then, the resulting culture was centrifuged to separate the cell debris and concentrated by 8% polyethylene glycol 6000 (19). In the next step, it was deactivated by 4 mM w/v per liter Binary Ethylenimine (BEI) for 30 h at 30°C. To neutralize and remove residuses and 2 mM (sodium thiosulfate) was added (10). Finally, 2.7 × 107 TCID50 of inactive virus (O2016/IR) was used for each vaccine dose.
3.5. Formulation
- Combined vaccines containing aluminum hydroxide (Gel) 20% adjuvant:
(A) 2 mL vaccine: Aluminum hydroxide (gel) 20% + Enterotoxemia toxoid + PBS
(C) 2 mL vaccine: Aluminum hydroxide (gel) 20% + FMD vaccine + PBS
(E) 2 mL vaccine: FMD + Enterotoxemia + Aluminum hydroxide (gel) 20% adjuvant + PBS
Groups A, C, and E: Adjuvant added to the aqueous phase of vaccine bulk, mixed for 1 h in a low-speed agitating mixer at 20°C.
- Combined vaccines containing ISA 206 adjuvant:
(B) 2 mL vaccine: Montanide ISA 206 adjuvant (SEPPIC France) 50% + Enterotoxemia toxoid vaccine + PBS
(D) 2 mL vaccine: Montanide ISA 206 adjuvant (SEPPIC France) 50% + FMD vaccine + PBS
(F) 2 mL vaccine: FMD (O) + Enterotoxemia + Montanide ISA 206 adjuvant (SEPPIC France) 50% + PBS
Groups B, D, and F: The mixture was stirred at 300 rpm for 10 min at 30°C in a water incubator to form a water-in-oil-in-water blend (20)
G vaccine: 2 mL enterotoxemia toxoid vaccine (21)
H vaccine: 2 mL commercial enterotoxemia vaccine (control+)
I vaccine: 2 mL commercial FMD vaccine (control +)
J vaccine: Unvaccinated control animal (control -)
3.6. Experimental Design
We randomly divided 40 healthy sheep free from FMD and enterotoxemia into 10 groups of four sheep each and kept them separately in a controlled area. Antigen preparation was done in equal parts to one dose of commercial vaccine (22-24). Immunization of sheep's performed as two subcutaneously (SC) administrations with two-week intervals in selected groups (A-J). Blood samples of about 10 mL were drawn from every animal within one year after the last immunization. The samples were centrifuged at 2,500 rpm for 15 min, and the sera were kept at -20°C till future evaluation (5, 8, 24).
3.7. Indirect ELISA
Toxin solution was prepared by carbonate buffer with a dilution of 1:1000. Then, 50 microliters of it was poured into microplate wells and incubated overnight at 4°C.Plate were washed Four times for two minutes by washing solution (a solution of PBS 1X and Tween 20) was washed and treated with 100 microliters of 1% BSA solution (0.01 μg/mL), and it was blocked for 30 minutes. In the next step, the test antisera were diluted with a dilution solution of 1:150, the negative control with a dilution of 1.40, and the positive control with a dilution of 1.400. Then, 100 microliters of different serum samples were poured into the rows of A1-12. Also, 50 microliters of the diluting solution (a solution of 1X PBS and 1% BSA) were poured into all wells in parts A1-12. Then, 50 microliters were taken from the wells of row A1-12 and poured into the wells of row B1-12 and completely pipetted and poured into the wells of C1-12. The same operation was done for the next rows, and finally, the last 50 microliters were thrown away, and the plate was incubated for 60 minutes. After the desired time, washing was done as in the second step. Then, 50 microliters of Goat Anti-Rabbit IgG Antibody-HRP conjugate (with a dilution of 1.6000) were added and incubated for 60 minutes in the dark. After the desired time, the plate was washed five times, and 50 microliters of 3,3',5,5'-Tetramethylbenzidine TMB were poured into the wells and placed in the dark for 15 minutes. In the last step, we added 50 microliters of 1 M hydrochloric acid solution and immediately measured the OD (optical absorption) with an ELISA reader (Biotech Company) at a wavelength of 450 nm. Then, the results were analyzed (11).
3.8. Serum Neutralization Test
To perform the Serum Neutralization Test (SN) to check the virus antibody titer, animal serum samples were placed in a water bath at 56°C for 30 minutes (according to the method recommended by the World Organization for Animal Health (OIE) manual). Subsequently, two-fold serial dilutions of the serum were mixed with purified FMDV (serotypes O/2016/IR) suspension containing 100 TCID50 (50% tissue culture infective dose) in a microtiter plate. The mixture was incubated at 37°C in 5% CO2 for 1 h. A 50 µL of BHK-21 (106 cells/mL) grown on DMEM (Gibco, USA) and 10% Fetal Calf Serum (FCS, Invitrogen) was added to each well. The microtiter plate was then incubated at 37°C in a 5% CO2 atmosphere for 72 h and expressed as the reciprocal of the dilution that neutralized 50% of the virus in BHK-21 cells. Neutralizing antibodies titers were calculated as log10 geometric mean titers (24, 25).
3.9. Statistical Analysis
Tukey's test was used to compare mean values between vaccine groups at each month using linear contrasts for the post hoc analysis. The significance level was set at α = 0.05. Using repeated-measures ANOVA, we compared the SI values between the groups that received the vaccine alone and those that received the combined vaccine. The level of significance was set at P < 0.05.
4. Results
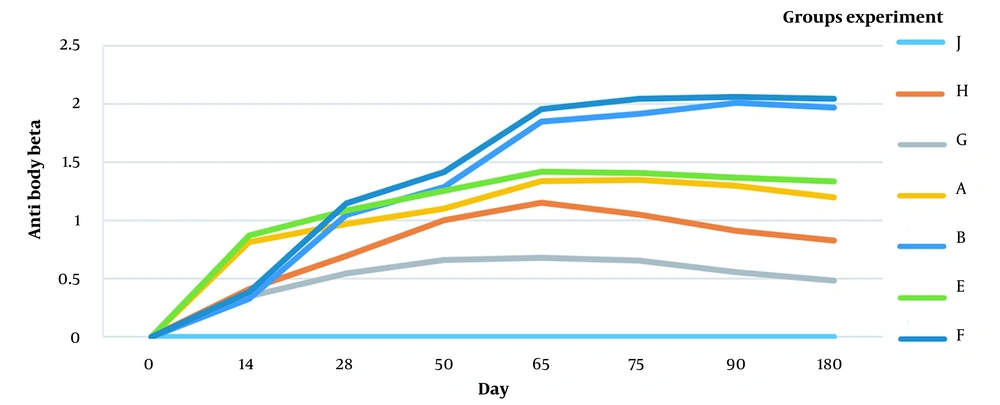
The following figures compare antibody titer against the FMD virus and Clostridium perfringens toxin (beta and epsilon) (Figures 3, 4, and 5).
Protective antibody titer against serotype (O) of foot and mouth disease (cut-off > 1.2), titer less than 0.1 was not calculated. J (control-), I (commercial vaccine, control+), E (toxoid + FMD + aluminum hydroxide), F (toxoid + FMD + ISA206), C (FMD + aluminum hydroxide), D (FMD + ISA206).
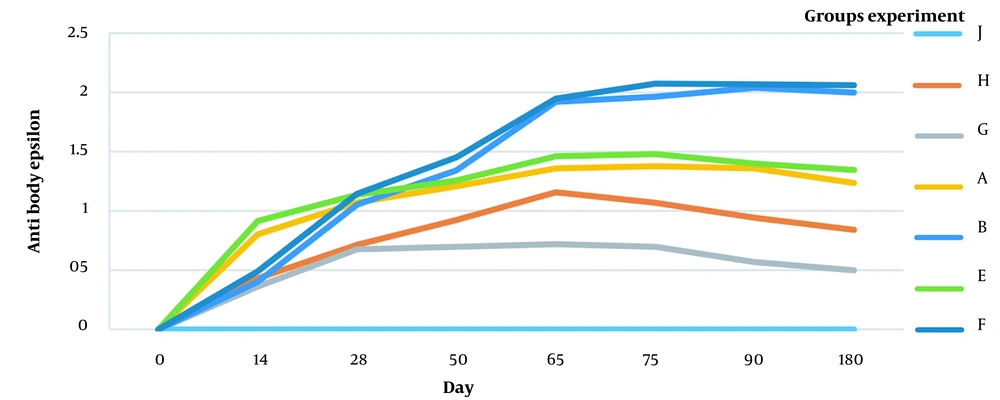
4.1. Humoral Immunity Against Beta Toxin and Epsilon Toxin
The antibody titer (beta and epsilon toxins) is shown in Figures 3 and 4. The antibodies against beta and epsilon toxins gradually increased in all immunized groups. The animals vaccinated with combined aluminum hydroxide (gel) showed a faster increase than the ISA 206 vaccinated ones. However, antibody production levels and duration were higher in ISA 206 immunized animals. As shown in Figures 3 and 4, the ratio of specific antibodies against beta and epsilon toxins significantly differed between group toxoid + FMD + ISA206 (1.579 ± 0.028) and group toxoid + FMD + Aluminum hydroxide (1.247 ± 0.034) (P < 0.05). This indicates that ISA206 was more effective than aluminum hydroxide for neutralizing beta and epsilon toxins. The results suggest that the toxoid vaccine required an adjuvant to increase immunogenicity because group G had a lower titer of epsilon and beta antibodies than other groups.
4.2. Neutralizing Antibodies Derived from Vaccine Against FMDV
Figure 5 shows the titer of the protective antibody (according to OIE 1.2>). The serum neutralization test (SN) and the formula (read and monarch) calculated the sera's logarithmic titer of the antibody response. It was shown that the vaccinated groups had a protective titer. The foot-and-mouth disease antibody titer reached the highest level on the 65th day in the vaccination groups immunized with aluminum hydroxide (C and E groups) and on the 75th day in the vaccinated groups with ISA206 (F and D groups). A significant difference was seen in the protective titers of aluminum hydroxide and ISA206. This indicates that ISA206 had a higher immunogenicity and was more effective (P <0.05).
5. Discussion
Vaccination with high immunogenicity reduces clinical symptoms caused by bacterial or viral infections. Recently, studies have been done on combining bacterial-viral vaccines to improve formulation methods and minimize cost and stress in livestock, increasing vaccine efficacy. In 2021, Muenthaisong et al. injected cattle with a mixed bacterial-viral vaccine (FMD and HS), with the group receiving the combined vaccine (FMD-HS) having a higher antibody titer than the group receiving each vaccine alone (20, 26). The combined vaccine of BI-Bagoury et al. on livestock from inactive FMD and BEF viruses was tested in 2014 with ISA206 adjuvant, which showed immunity to animals after the second injection without interfering with the immune response of the antigens (12). In 2016, Park et al. injected the FMD vaccine with ISA206 oil adjuvant and aluminum hydroxide into mouse and goat models. The results indicated that the group vaccinated with ISA206 adjuvant had better results in their challenge (13), and their antibody titer was higher than that in the combined gel and oil vaccine groups. The results of the FMD vaccine with adjuvant ISA206 by Shabana et al. in 2018 showed that this vaccine could be immunogenic for one year (5).
Evaluating C. perfringens toxoid with ISA206 adjuvant and aluminum hydroxide gel on rabbit and goat models showed that the ISA206 toxoid vaccine was better than the toxoid vaccine and aluminum hydroxide toxoid (8). Oil adjuvants are based on W/O/W emulsion, and the antigen is in the aqueous phase. In this type of adjuvant, the antigen is released slowly, which means that in addition to short-term immunogenicity, they cause long-term immunity. In this study, the highest antibody level on the 75th day is in animals vaccinated with ISA206 adjuvant (Figure 3, 4, and 5). The formulation of the vaccine containing ISA206 oil adjuvant produces a stronger response against bacteria and viruses and increases the length of the immunogenic period. It has been demonstrated that combined vaccines instead of monovalent vaccines can improve vaccine quality while saving money and time; they are good options for the industrial phase and mass production of FMDV and enterotoxemia toxoids and reduce the need for revaccination.
5.1. Conclusions
The results of this study demonstrated that vaccines (C. perfringens toxoid and FMDV) administered with the ISA 206 adjuvant had higher immunogenicity than aluminum hydroxide-based adjuvants. ELISA and virus neutralization test (VNT) results obtained in sheep immunized with aluminum hydroxide, and ISA 206 vaccines demonstrated that the combination of immunogens produced a long-term and stable immune response without interfering with FMD and enterotoxemia immunity. Production of new vaccines has an export value and is a suitable tool for generating foreign exchange income for the country. Approximately 40 million doses of FMDV and enterotoxemia vaccines are consumed in Iran every year, which can be distributed with a new multivalent viral and bacterial vaccine against these two diseases that can significantly damage this industry. For the industrial production of such vaccines, more investigation is needed to establish other possible interactions and necessary infrastructure for vaccine production.