1. Background
Nocardia is a partially acid-fast, spore-free, catalase-positive, rod-shaped bacterium frequently isolated from clinical samples, particularly in immunocompromised patients (1). These widely environmentally distributed bacteria are responsible for infections such as pulmonary and cutaneous nocardiosis, abscesses, cellulitis, and mycetoma. The infection can disseminate to the brain, kidneys, joints, heart, eyes, and bones (2). One of the primary challenges in the clinics is difficulty in the timely diagnosis of nocardiosis due to the lack of specific pathognomonic clinical signs (3). Direct microscopic examination (DME) and streak plate methods are laborious and time-consuming. So, colony morphological features are variable, and Nocardia species may be mistaken for members of closely related genera acid-fast bacilli (AFB) (4). Nucleic Acid Amplification Tests (NAATs), such as polymerase chain reaction (PCR) with high specificity and sensitivity, have been established. However, the molecular method requires a thermal cycler machine, which is impossible for laboratories in deprived parts (5, 6). So, the assay requires post-PCR electrophoresis to detect the amplified amplicons, which can be labor-intensive and time-consuming (3, 7).
It has recently been found that loop-mediated isothermal amplification (LAMP) is a fast, affordable, easy, straightforward, and simple method of amplifying nucleic acids. Under isothermal conditions, Bst DNA polymerase performs an auto-cycling strand displacement DNA synthesis. It has been described and applied in microbiological diagnosis (8, 9). The following are features of LAMP: (i) all reactions can be carried out under isothermal conditions; (ii) the precision of the reaction is very high because it uses four primers identifying six separate regions on the target genome; (iii) amplification can be accomplished in a reduced time compared to amplification by PCR because there is no loss of time from thermal cycling; (iv) it produces substantial amounts of amplified products and allows simple detection approaches such as visual decision based on the reaction mixture's turbidity or fluorescence, which is maintained in the tube (10). In addition to these features, LAMP of DNA has been developed as a reliable method for identifying patient pathogens. Recently, Nagamine et al. (11) reported that the LAMP reaction time could even be less than half of that for the original LAMP technique when two additional primers, referred to as loop primers, were added (12).
2. Objectives
To the best of our knowledge, this is the first study of clinical samples to evaluate the Nocardia genome using the LAMP technique. The present study was designed to develop and evaluate a simple and quick method based on a LAMP assay for detecting Nocardia spp isolated from bronchoalveolar lavage (BAL) samples.
3. Methods
3.1. Bacterial Strains
In this cross-sectional study, 357 BAL samples were collected from patients referred to the two teaching hospitals. It is important to note that most of the BAL samples were collected for our previous study (12, 13). According to Eshraghi and Amin, the decontamination process may inhibit the growth of Nocardia, so the samples were not treated with any chemicals. However, a centrifuge was required to concentrate the lavage. In this regard, BAL samples were centrifuged at 1,500 × g for 10 minutes. Supernatants were removed, and examinations were performed on the deposits (14). Sabouraud dextrose agar (cycloheximide and antibiotic-free) was used for cultivation. To initially identify Nocardia species, Kinyoun acid-fast and partially acid-fast from colonies were performed as previously described (15, 16).
3.2. Molecular Studies
3.2.1. DNA Extraction
Genomic DNA was extracted from clinical samples using a CinnaGen DNA extraction kit (Cinnagen, Tehran, Iran) according to the manufacturer's instructions. The absorbance ratio (ODA260/280 = 1.8 - 2.0 nm) was used to assess the purity of cellular DNA.
3.2.2. PCR Assay
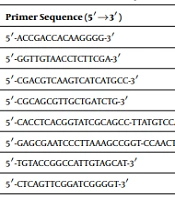
The molecular detection of isolates was performed using PCR-based amplification for a 596 bp fragment of the 16S rRNA gene, as Laurent et al. (7) suggested. The sequences of primers used in this study are listed in Table 1. The reaction mixture contained 2 µL of 10 × PCR buffer, 0.8 µL of MgCl2, 0.6 µL of dNTPs, 1.5 ng/mL DNA template, and 0.7 IU of Taq DNA polymerase (Ampliqon Co., Denmark). Primers were used to amplify Nocardia's DNA through a high-throughput PCR on a 1.5% agarose gel stained with safe stain (Sinaclon, Iran). The DNA amplification tool Master cycler gradient was used for the procedure (Eppendorf, CO, Germany) at 95°C for four minutes. A 1 kbp DNA marker (Fermentase Co., USA) was used as a size reference for the PCR method.
| Target Gene | Primer Sequence (5′→3′) | Reference |
|---|---|---|
| NG1 | 5′-ACCGACCACAAGGGG-3′ | (7) |
| NG2 | 5′-GGTTGTAACCTCTTCGA-3′ | |
| F3 | 5′-CGACGTCAAGTCATCATGCC-3′ | This study |
| B3 | 5′-CGCAGCGTTGCTGATCTG-3′ | |
| FIP | 5′-CACCTCACGGTATCGCAGCC-TTATGTCCAGGGCTTCACAC-3′ | |
| BIP | 5′-GAGCGAATCCCTTAAAGCCGGT-CCAACTTCACGGGGTCGA-3′ | |
| LF | 5′-TGTACCGGCCATTGTAGCAT-3′ | |
| LB | 5′-CTCAGTTCGGATCGGGGT-3′ |
Sequences of Oligonucleotide Primers Used in This Study
3.2.3. Primer Design
A set of inner (forward inner primer (FIP) and backward inner primer (BIP)), outer (F3 and B3), and loop primers (LF and LB) were designed for the LAMP assay to target eight separate regions. The protocol was intended to detect Nocardia strains using the species' target 16S rRNA. Using LAMP, a group of six primers was designed to target eight various regions. Constituting FIP was a complementary sequence of F1 (F1c), a T-T-T-T linker, and F2, while constituting BIP was a complementary sequence of B1 (B1c), a T-T-T-T linker, and B2. The loop primers LF and LB are situated between the regions of F2 and F1 or B1 and B2, respectively, as opposed to the outer primers F3 and B3, which are outside of the regions of F2 and B2. The Tm (temperature dissociation), stability of the final primer sequence, guanine-cytosine content, secondary structure creation, and distance between the primers were considered when creating LAMP primers using the primer explorer V4 program (http://primerexplorer.jp/).
3.2.4. LAMP Method
A loop amp DNA amplification kit (Eiken Chemical Co., Ltd., Tochigi, Japan) was used for the LAMP reaction. The LAMP was performed in a whole volume of 25 µL reaction mixture comprising 2 µL of each FIP and BIP inner primer, 1 µL of the F3 and B3 outer primers, 1 µL of LF and LB of loop primers, 2 mM dNTPs mixture (Takara Biotechnology, Dalian, China), 1.5 mM MgSO4, 2 mM betaine (Sigma, St. Louis, MO, USA), 2 mM Thermopol buffer (New England Biolabs, Ipswich, MA, USA), 3 µL of template DNA, and 6.5 mM ddH2O. The mixture was heated at 95°C for 5 min and then chilled on ice. Then, 8 U Bst DNA polymerase large fragments were added, followed by incubation at 65°C for one hour and heating at 80°C for 10 min to terminate the reaction.
3.2.5. Analysis of LAMP Products
A ladder-like pattern was observed through electrophoresis on a 2% agarose gel and visualized with a UV transilluminator. Staining the products with SYBR Safe (InvitrogenTM) fluorescent dyes evaluated the best observation mode. When LAMP products are present, the solution turns green, while without any amplification, it remains orange.
3.2.6. Specificity of LAMP
For determination of the method's specificity, LAMP was carried out with genomic DNA templates from Nocardia asteroides ATCC 19247, N. brasiliensis ATCC 19296, N. farcinica ATCC 23826, and non-Nocardia strains, including M. tuberculosis H37RV, Escherichia coli ATCC 25922, Enterococcus faecalis ATCC 29219, Bacillus cereus ATCC 14579, and Acinetobacter baumannii ATCC 19606.
3.2.7. LAMP Sensitivity
A 10-fold serial dilution of extracted DNA was used as a template for LAMP following the predetermined conditions defined above. A tube without DNA was included as a negative control. The amplicons were visualized on the 2% agarose gel. A serial dilution of genomic DNA was subjected to PCR to compare the detection sensitivity of LAMP and PCR. The sensitivities of LAMP and PCR were directed in triplicate, and the detection limit was defined as the last positive dilutions.
3.2.8. Applicability of LAMP
The LAMP results were compared with microscopic (partially acid-fast), microbiological-biotechnical, and conventional PCR results to evaluate the accuracy of the technique for diagnosing Nocardia spp.
4. Results
4.1. Bacterial Isolates
Out of 357 BAL samples, 0 (0.0%), 4 (1.1%), and 9 (2.5%) Nocardia strains were identified by the partial acid-fast staining, microbiological culture, PCR, and LAMP methods, respectively. The population under study had a mean age of 49 ± 1.5 years, ranging from 17 to 89 years. The isolates were obtained from patients of different age groups: 12 - 22 years N = 45, 22 - 32 years N= 49, 33 - 43 years N = 68, 44 - 54 years N = 59, 55 - 65 years N = 55, 66 - 76 years N = 45, and 77 - 89 years N = 36. Also, 221 (61.9%) patients were male, and 136 (38.1%) were female.
4.2. Detection and Confirmation of LAMP Products
Many ladder-like bands were identified by agarose gel electrophoresis after a successful LAMP reaction with species primers. Also, color changes from orange to green were observed by adding calcium and manganese ions. After the addition of SYBR Safe, the recognized conditions allowed the imagining of the amplified products under UV light.
4.3. Sensitivity of LAMP
For Nocardia spp, PCR and LAMP sensitivities were 2.0 CFU/reaction and 191 CFU/reaction, respectively. Compared to PCR, the LAMP had a 100-fold higher sensitivity.
4.4. Specificity of LAMP
No reactions were observed when the non-Nocardia genome was used in the LAMP assay. However, reactions were observed when Nocardia isolates were used in the LAMP assay. These results approved that the 16S rRNA sequences (NG) targeted are specific for Nocardia (7).
5. Discussion
The present study used partial acid-fast staining, culture, PCR, and LAMP methods to identify Nocardia strains in clinical samples. We found 0 (0.0%), 4 (1.1%), 9 (2.5%), and 10 (2.8%) Nocardia strains by microscopic examination, streak culture plate, PCR, and LAMP, respectively. Habibnia et al. (17) showed that among 300 soil samples cultured with the paraffin baiting method, 57 (19%) isolates were identified with initial tests. This difference may be due to the sample source (clinical sample vs. environmental). Fatahi Bafghi et al. (18) showed that out of 517 clinical samples, seven, five, and three Nocardia spp were isolated using the paraffin baiting technique, paraffin agar technique, and SDA with and without cycloheximide, respectively.
The assay created can address several issues with the bacteriological diagnosis of nocardiosis in addition to being specific, sensitive, and fast (19). Due to laborious phenotypic approaches, nocardiosis is occasionally identified after spreading to other tissues or a patient's death (19, 20). So, the beginning of proper therapy is supreme in decreasing the high mortality and morbidity frequency of patients with this infection and is reliant on early recognition of the Nocardia disease (21, 22). A confirmed method for identifying Nocardia is isolating bacteria from samples, but it is supposed to lack sensitivity (23, 24). Although Nocardia isolates can grow on many types of medium, it has been challenging to separate them from the complex mixed flora of clinical samples (mostly lung specimens like BAL) because the strains grow more slowly than the other contaminating microbes (25, 26). Empirical therapy before sampling can also prevent or delay microbial growth, particularly in the skin or cerebral abscesses (27). Despite various other contaminating or colonizing organisms in samples, the molecular approach overcomes these challenges through the targeted amplification of nocardial genomic DNA.
These techniques enabled the identification of bacteria even after the onset of intensive chemotherapy since they were unaffected by the organism viability. For quick and accurate identification, molecular-based techniques like PCR have been developed; nevertheless, it is frequently essential to employ a thermal cycle (28). So, real-time PCR has been applied to detect pathogens. However, it is infrequent due to its expensive thermal cycler and practical expertise needs, and labor-intensive procedure (29). Therefore, we established a novel Nucleic Acid Amplification Test (NAAT), LAMP, for detecting Nocardia spp, either as culture isolates or in samples. Our study detected 9 (2.5%) and 10 (2.8%) Nocardia isolates using PCR and LAMP methods. Possible explanations for the lower detection rate by conventional PCR include low copy numbers of nocardial genomic DNA templates or the presence of specific PCR inhibitors that affect reaction sensitivity.
Our results showed that the LAMP method is specific, sensitive, and efficient. As a popular NAAT, LAMP was known for being quick, specific, sensitive, profitable, and user-friendly. Furthermore, the LAMP's positive or negative findings can be simply assessed with the bare eye (white precipitation or fluorescence intensity under UV illumination). Visual inspection of the materials after the LAMP reaction, which takes 45 - 60 minutes, yields the results. The LAMP method required only a simple instrument, a water bath, to achieve high sensitivity and specificity (97.4% and 100%, respectively). The results corroborating Wang et al. (30) and Zhang et al. (31) showed that the LAMP method was 100 times more sensitive than the PCR when using bacterial cellular DNA as a template.
5.1. Conclusions
We developed a novel, rapid, sensitive, straightforward, and inexpensive LAMP method for detecting Nocardia. Monitoring for contamination of these bacteria in samples should be made simpler by LAMP identification of Nocardia spp. To the best of our knowledge, this is the first account of the LAMP method being used to identify Nocardia spp in BAL samples. The LAMP assay was set up in both pure culture and direct detection from samples in the current study. Furthermore, the LAMP method helps detect Nocardia strains in different clinical samples.