1. Background
Oropharyngeal candidiasis (OPC) is one of the most common infections among the HIV-infected population (1). Oropharyngeal candidiasis is a useful indicator of HIV progression following the failure of highly active antiretroviral therapy (HAART) (2). Candida albicans is the most frequent Candida species causing OPC in these patients (3), and the oral cavity can act as a potential source of systemic fatal candidiasis (4). The pathogenesis of Candida is mediated by several virulence factors, including biofilm formation and oxidative stress response (5).
Catalase, superoxide dismutase (SOD), and glutathione peroxidase (GPX) are three enzymes involved in protecting cells from the harmful effects of reactive oxygen species (ROS) (6, 7). Reactive oxygen species are especially important in pathogenic fungi and have been emphasized in C. albicans because oxidative stress is a tense condition more likely to be encountered in vivo (8). The activity of SOD produces hydrogen peroxide (H2O2), and the capacity of yeast to develop tolerance against H2O2 depends upon catalase encoded by the CAT1 gene (9). Also, six master transcriptional regulators, including BCR1, EFG1, TEC1, NDT80, ROB1, and BRG1, regulate the formation of C. albicans biofilm (10). Biofilm acts as a source for chronic and recurrent infections and is responsible for the emergence of antifungal drug resistance, which is caused by an extracellular matrix limiting the penetration of drugs; only the surface cells of a biofilm are exposed to an effective dose of the antifungal agent (11, 12). There is a need to develop newer anti-biofilm compounds and modify the existing antifungals that affect the biofilm formation by Candida species to make them more effective.
Peganum harmala L. is among the traditional medicinal plants growing in semi-arid climates, steppe areas, and sandy soils. Capsules containing > 50 small black-brown triangular seeds constitute this plant’s fruit. For a long time, different parts of P. harmala, especially its seeds, have been used in Iran as a traditional medicine for various diseases (13). The main medicinal properties of P. harmala are attributed to the production of alkaloids in different parts of this herb, especially seeds, and roots (14).
2. Objectives
Since the results of our previous study confirmed the theory of the effect of P. harmala extract (PHE) on biofilm formation (15), in the present study, an attempt was made for the first time to identify the mechanism of anti-biofilm properties of PHE by investigating the expression of CAT1 gene, which is involved in oxidative stress response pathway, as well as morphogenetic regulator gene (EFG1) and cell wall regulator gene (BCR1) as key genes for biofilm formation.
3. Methods
3.1. Specimen Collection and Initial Identification
This research was conducted on 33 C. albicans strains isolated from HIV-positive individuals in the Behavioral Diseases Counseling Center affiliated to Ahvaz Jundishapur University of Medical Sciences. The inclusion criteria were: Oral candidiasis symptoms such as white patches, loss of taste, and a painful burning sensation in the mouth in HIV-positive patients. Samples were collected from the oral cavity of the participants using a sterile cotton swab. The swabs were then inoculated onto CHROMagar Candida plates (CHROMagar Candida, France) and incubated aerobically at 37°C for 48 hours. Patients with ≥ 10 CFU yeasts grown from each swab on the plate were considered to be colonized (16).
3.2. Molecular Identification
DNA was extracted from purified colonies by the boiling lysis method and subjected to polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). The ITS1-5.8S rDNA-ITS2 region was amplified using universal fungal primers ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) (17). Subsequently, PCR products were digested by MspI restriction enzyme (Hpa III; Thermo Fisher Scientific, Waltham, MA, USA). After incubation for 16 hours at 37°C, the products were transferred to 1.8% agarose gel. Due to the inability of the MspI enzyme to distinguish between C. albicans and C. dubliniensis, the suspected C. albicans and C. dubliniensis isolates were differentiated by duplex PCR using CAL (F: 5′-TGG TAA GGC GGG ATC GCT T-3′, R: 5′-GGT CAA AGT TTG AAG ATA TAC-3′) and CDU (F: 5′AAA CTT GTC ACG AGA TTA TTT TT3′, R: 5′AAA GTT TGA AGA ATA AAA TGG C-3′) primers (18) as shown in Figure 1.
3.3. Plant Preparation
Peganum harmala seeds were collected from Iran on December 2020, and a voucher specimen was deposited at the Medicinal Plant Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (code no. A233230001sp). After collecting the plants, they were rinsed with distilled water and dried for investigation of antifungal activity.
3.4. Preparation of Plant Extract
We used the cold maceration method for extraction. Ten grams of fine powder from P. harmala seeds were macerated using 100 mL of 20% ethanol in a closed conical flask for each case on a rotary shaker (150 - 180 r.p.m) for 24 hours, which was then filtered using Whatman filter paper No.2. The solvent-free ethanol extract was after that evaluated (19).
3.5. In Vitro Biofilm Formation Assay
Tubes containing 1.0 mL of SDB medium (Sabouraud Dextrose Broth-Liofilchem, Italy) and C. albicans were incubated in a shaking incubator at 30°C overnight. After incubation, C. albicans cells were rinsed three times with phosphate-buffered saline (PBS), and the optical density of C. albicans cell suspension (1.0 × 106 cells/mL) was determined by spectrophotometry at 530 nm (Abs.530). In vitro, biofilm formation assay was performed on 96-well microplates based on the method described by Pierce et al. with minor modifications (20). In brief, all the standardized cell suspensions were diluted at a ratio of 1:10 in RPMI 1640 medium (with L- glutamine, without bicarbonate) (Bio Basic) and buffered to pH = 7.0 with 0.165 M solution of MOPS (Bio Basic). Afterward, 200 μL of cells (1.0 × 106 cells/mL) was seeded into 96 sterile flat-bottom well microplates and incubated at 37°C for 24 hours until mature biofilm formation.
3.5.1. Crystal Violet Assay for Determination of Biofilm Formation
To remove the planktonic cells, the plates were gently washed three times with PBS, and biofilm biomass was stained by adding 0.1% crystal violet (100 μL) for 15 minutes. The wells were then washed three times with PBS to remove the excess stain and dried at ambient temperature. Subsequently, 100 µL of ethanol was added to each well. After these steps, the biofilm biomass was measured with an ELISA Microplate Reader at 595 nm. Crystal violet assay was conducted in triplicates for C. albicans ATCC 10231 (the common positive control for evaluating anti-biofilm agents) and each clinical isolate.
3.6. Evaluation of the Anti-biofilm Activity of PHE
Following mature biofilm formation (24 h) and after washing the wells, as previously mentioned, serial dilutions of PHE were added to wells with concentrations of 0.245 - 62.5 ug/uL in RPMI 1640 medium. The plates were then incubated at 37°C for 24 h to determine the anti-biofilm effects of PHE (21) compared with 20 μg/mL Amphotericin B (AmB) and the negative control. After incubation, minimum biofilm eradication concentration (MBEC) was defined as the lowest PHE concentration required to eliminate 99.9% of biofilm-embedded yeast (3 log10 reductions in CFU/mL) compared to growth control and negative control (22, 23). For this purpose, a well in which no biofilm was observed compared to the growth control and the negative control was defined as MBEC.
3.7. RNA Extraction and Real-time PCR
A total of 15 isolates were selected for expression analysis of target genes based on the intensity of the effect of the herbal extract on the degradation of biofilm from Candida species. To compare the changes in expression levels of CAT1, BCR1, and EFG1 genes in C. albicans isolates before and after treatment with MBEC of PHE, the biofilm of C. albicans isolates was simultaneously formed in two separate plates (24-well, treated, flat-bottom, non-pyrogenic, sterile) for the selected isolates. In the first plate, the biofilm was collected without exposure to PHE in 1.5 mL microtubes. The biofilm was exposed to one concentration less than MBEC of PHE in the other plate and collected in 1.5 mL microtubes (23). Subsequently, total RNA was extracted from C. albicans isolates in RNase-free conditions using RNX-PlusTM solution following the Sinaclon total RNA isolation protocol. RNA concentrations were measured using a spectrophotometer (NanoDrop, Thermo Scientific). Synthesis of cDNA was performed by 2X RT Master Mix of No-ROX kit (BiofactTM) based on the manufacturer’s instructions, and the β-Actin gene was considered as the normalizing gene. The primer sequences of β-Actin, EFG1, CAT1, and BCR1 genes are shown in Table 1. Quantitative real-time PCR was performed in triplicate using real-time PCR Roche LightCycler® with the following parameters: A pre-incubation step at 95°C for 5 minutes, followed by 40-cycles of 95°C for 15 seconds (denaturation) and 60°C for 60 seconds (annealing and extension).
| Genes | Primer Sequence (5′→3′) | Size (bp) | Gene Bank | GC% | TM | References |
|---|---|---|---|---|---|---|
| BCR1 | 101 | NC_032096.1 | (24) | |||
| F | CTTCAGCAGCTTCATTAACACCTA | 41.7 | 68 | |||
| R | TCTTGGATCAGGTGTACTTTTCAA | 37.5 | 66 | |||
| EFG1 | 100 | XM_709144.2 | (25) | |||
| F | TGCCAATAATGTGTCGGTTG | 45 | 58 | |||
| R | CCCATCTCTTCTACCACGTGTC | 54 | 68 | |||
| CAT1 | 117 | NC_032089.1 | (26) | |||
| F | GACTGCTTACATTCAAAC | 38.9 | 55.1 | |||
| R | AACTTACCAAATCTTCTCA | 31.9 | 55.1 | |||
| ACT1 | 166 | XM_019475182.1 | ||||
| F | ACTGCTTTGGCTCCATCTTCT | 48 | 65 | |||
| R | TGTGGTGAACAATGGATGGAC | 48 | 62 |
The Nucleotide Sequence of the Primers for Real-time PCR Amplification
3.8. Statistical Analyses
Statistical analysis of quantitative real-time PCR was carried out using Microsoft Excel workbook, relative expression software (REST 2009), and Graph Pad PRISM 8. The mean Ct of EFG1, CAT1, and BCR1 genes before and after treatment by PHE was expressed as mean ± standard deviation. Then, using paired t-test, the levels of ΔCt of EFG1, CAT1, and BCR1 genes were compared separately before and after incubation with the herbal extract. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Biofilm Formation
Candida albicans species were successfully isolated and identified. The ability of biofilm formation in C. albicans isolates was evaluated according to the crystal violet assay. The intensity of biofilm formation was 0.18 - 4.8 according to the absorbance values at 595 nm (Table 2).
| Intensity of Biofilm Formation a | No. (%) |
|---|---|
| < 1 | 4 (12.1) |
| 1 - 2 | 7 (21.2) |
| 2 - 3 | 9 (27.3) |
| 3 - 4 | 3 (9.1) |
| > 4 | 10 (30.3) |
The Intensity of Biofilm Formation of 33 Candida albicans Isolates
4.2. Evaluation of the Anti-biofilm Effect of PHE
Peganum harmala extract showed a strong antifungal effect against C. albicans isolates. Biofilm reduction under the influence of PHE was observed macroscopically in all biofilms, and MBEC was obtained in 15 isolates (33%) in the n 0.49 - 7.8 μg/mL concentration range of PHE (Table 3). Also, for all tested isolates, biofilm reduction was achieved under the influence of 20 μg/mL AmB.
| Isolate Name | Biofilm Formation | MBEC (μg/mL) |
|---|---|---|
| H007 | 4.7 | 1.95 |
| H031 | 4.7 | 0.98 |
| H024 | 4.6 | 0.98 |
| H028 | 4.0 | 0.98 |
| H072 | 3.7 | 3.9 |
| H025 | 3.4 | 7.8 |
| H021 | 2.7 | 1.95 |
| H043 | 2.5 | 0.49 |
| H079 | 1.9 | 3.9 |
| H026 | 1.4 | 0.98 |
| H078 | 1.1 | 3.9 |
| H046 | 1.0 | 0.98 |
| H059 | 0.77 | 1.95 |
| H075 | 0.7 | 1.95 |
| H008 | 0.18 | 3.9 |
The Intensity of Biofilm Formation of 15 Candida albicans Isolates and Anti-biofilm Activities of Peganum harmala Against These Isolates
4.3. Expression evaluation of CAT1, EFG1, and BCR1 Genes in Candida albicans Isolates
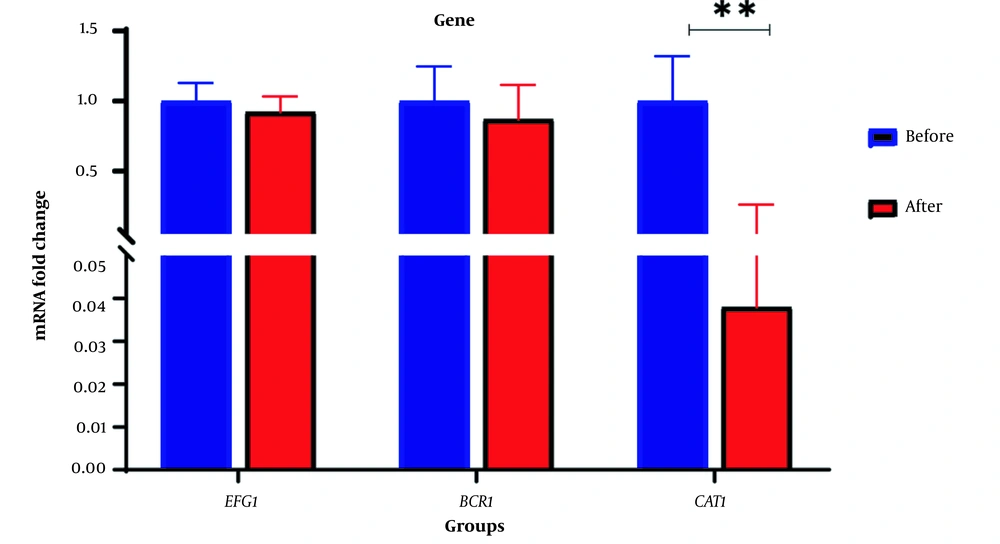
RNA extraction from biofilm was successfully performed before and after the treatment with one concentration less than MBEC of PHE. Real-time PCR was run using specific primers (CAT1, EFG1, and BCR1) and β-Actin as the internal control gene. Statistical analysis showed that biofilm formation was reduced in C. albicans cells exposed to PHE, after which the expression of CAT1 mRNA in C. albicans isolates was significantly down-regulated (P = 0.05). Although these treatments indicated a reduction in the expression of EFG1 and BCR1 genes (Figure 2), this difference was not statistically significant (Table 4). The minimum and maximum fold change in genes treated with PHE was related to CAT1 (0.038) and EFG1 (0.922) genes, respectively.
| Gene | Fold-Change Before Treatment Clinical Isolate | Fold-Change After Treatment Clinical Isolate | P-Value |
|---|---|---|---|
| CAT1 | 1 | 0.038 | 0.0068 |
| EFG1 | 1 | 0.922 | 0.5754 |
| BCR1 | 1 | 0.872 | 0.529 |
P-Value and Fold-Change Before and After the Treatment of Clinical Isolates
5. Discussion
Previous studies have demonstrated that biofilm drug resistance is a multifactorial phenomenon influenced by factors such as the extent of matrix formation, biofilm composition, and the simultaneous presence of bacteria in the biofilm, which can lead to clinical complications in antifungal therapies (27). Treatment failure indicates the importance of research on new antifungal compounds (28). Natural products such as medicinal herbs appear to be the most promising antifungal treatments (23). Peganum harmala L. is a medicinal plant with numerous documented pharmacological properties (29, 30). The main β-carboline alkaloids of P. harmala seeds are harmine (7-methoxy-1-methyl-9H-pyrido [3, 4-b] indole) and harmaline (4, 9-dihydro-7-methoxy-1-methyl-3H-pyrido [3, 4-b] indole) that play an important role in pharmacological properties of P. harmala (31). Hitherto, Aboualigalehdari et al.'s study is the only research investigating the anti-biofilm properties of P. harmala against C. albicans (15). We aimed to identify the anti-biofilm mechanisms of PHE. Therefore, the effect of PHE was evaluated against the biofilm from 33 C. albicans clinical isolates. Anti-biofilm properties of PHE were confirmed in most isolates, consistent with previous studies’ results (15, 32).
Mean fold change in the expression of EFG1 and BCR1 genes decreased before and after the treatment with the extract; however, contrary to expectations, our results did not demonstrate significant differences with p-values of 0.5754 and 0.529, respectively. CAT1 expression was significantly reduced in C. albicans biofilm treated with PHE (P = 0.0068). CAT1, the gene encoding the catalase enzyme, is essential in enhancing the tolerance against oxidative stress (33) and is required for ROS detoxification in C. albicans (34). CAT1 is also involved in the initial stages of C. albicans attachment to surfaces. Therefore, CAT1 mutants are not able to form a biofilm (35). Previous investigations show that antifungals affecting Candida biofilm, such as miconazole and AMB, affect ROS. Several studies have demonstrated that as well as inhibiting ergosterol biosynthesis, miconazole induces accumulation of ROS in C. albicans planktonic cells, possibly due to the inhibition of the enzymes involved in the degradation of peroxide radicals and H2O2 by miconazole (33, 36). One of these enzymes is catalase, which is involved in the breakdown of H2O2 (9). Peganum harmala extract is likely to accumulate H2O2 by reducing CAT1 expression and hence disturbing the pro-oxidant/antioxidant balance that leads to the overproduction of ROS, causing damage to cellular components and eventual destruction of C. albicans biofilm. These results may indicate that P. harmala and miconazole have similar anti-biofilm mechanisms.
Amphotericin B is another fungicidal that leads to the accumulation of ROS and apoptosis in C. albicans biofilm through interaction with ergosterol and subsequent pore formation (37). De Brucker et al. used SOD inhibitors to reinforce AmB activity against C. albicans biofilm (37). To the best of our knowledge, this study is the first research to identify the anti-biofilm mechanism of P. harmala; therefore, future studies should investigate the synergistic effects of PHE and AMB to enhance the anti-biofilm activity and the expression of SOD genes before and after treatment of C. albicans biofilm with PHE.
5.1. Conclusions
Our results demonstrated that PHE significantly decreased CAT1 expression in C. albicans cells treated with the herbal extract. PHE appears to accumulate H2O2 by reducing CAT1 expression, thus impairing pro-oxidant/antioxidant balance and leading to the overproduction of ROS, which can damage cellular components and eventually eliminate the C. albicans biofilm.