1. Background
Urinary tract infections (UTIs) are among the most prevalent infections in hospitals and communities worldwide (1). Urinary tract infection patients can be categorized into both symptomatic and asymptomatic patients. Patients with symptomatic UTIs are divided into 3 categories based on the severity of the disease: Cystitis (infection of the bladder), pyelonephritis (infection of the kidney), and urosepsis (2). In the United States, less than 12 million individuals with UTI are sent to health facilities each year, with 470 000 hospitalized, costing around $6 billion (3, 4). In uropathogenic Escherichia coli (UPEC), E. coli strains, which deviate from their commensal role as gut flora, develop and persist in the urinary tract and exhibit a wide range of virulence traits and tactics that allow them to infect the urinary tract and cause illnesses. These E. coli strains are referred to as UPEC because uropathogenicity is frequently associated with them (5).
The expression of a variety of virulence factors is linked to UPEC’s capacity to generate symptomatic UTIs (6). For E. coli, over 40 genes have been identified linked to the virulence of these bacteria (7). Some of these virulence factors are adhesins encoded by fimH (mannose-specific adhesins of type I fimbriae), toxins encoded by hly (hemolysin), sfa/foc (S-/F1c-fimbriae), papC and papG (P-fimbriae), afa (a fimbrial adhesin), iron acquisition factors produced by iucC (aerobactin iron transport system), cnf1 (cytotoxic necrotizing factor 1), ibeA (invasion of brain endothelium), and the virulence factor gene (VFG), neuC (sialic acid biosynthesis) (8). Some of these VFGs are organized into regions known as pathogenicity islands (PAIs), which stand out for their high expression and, in some circumstances, association with the generation of β-lactamases and antibiotic resistance (7). The mechanism for the coordinated horizontal transfer of virulence genes is provided by these virulence factors, which are frequently expressed in PAIs (9). The PAI idea was initially introduced by Hacker et al. in the late 1980 s (10).
Mobile genetic elements (known as PAIs) differ from the bacterial host genome’s insertion sequences, transposases, and integrases by having GC nucleotide content (11). These elements may range in size from 10 to 200 kb and encode genes linked to 1 or more virulence factors, such as invasions, poisons, adhesins, iron absorption, and secretion systems (12, 13). Uropathogenic E. coli strains are normally classified by serological typing of their O (lipopolysaccharide) antigen (14); in addition, serogroups O1, O2, O4, O6, O7, O8, O15, O16, O18, O21, O22, O25, O75, and O83 are usually expressed in UPEC clones (15, 16). Serogroup assays are used for precise E. coli identification and epidemiological research on E. coli epidemics (17). For the treatment of UTIs caused by UPEC, antibiotic therapy is often required (18), though, particularly in patients with recurrent UTIs, the worldwide spread of multiple drug resistance (MDR) bacterial strains has become a public health problem and is considered a serious health concern (19).
2. Objectives
Due to the medical importance of UTIs caused by UPEC, this study aimed to assess PAI markers, O-antigen serogroups, and resistance to antibiotic properties associated with UPEC isolates obtained from hospitalized patients in Rasht city hospitals.
3. Methods
3.1. Identification and Bacterial Isolation
A total of 110 urine samples were obtained from individuals with UTI referred to selected hospitals in Rasht, Iran. These samples were cultured in blood and MacConkey agar media for 24 hours at 37°C. The IMViC tests were used to identify E. coli. Polymerase chain reaction (PCR) amplification of the 16 S rRNA gene was used to validate the identification, as described elsewhere (20, 21). For each test, E. coli ATCC 25922 was used as the control strain.
3.2. Antibiotic Susceptibility Pattern
In accordance with the guidelines of the Clinical and Laboratory Standards Institute (CLSI), the Mueller Hinton agar (HiMedia, India) disc diffusion method of Kirby-Bauer was used to evaluate the antibiotic susceptibility pattern (22). The following antibiotics were used at the following concentrations: imipenem (10 µg), cefotaxime (10 µg), ceftriaxone (30 µg), ceftazidime (30 µg), gentamicin (10 µg), amikacin (30 µg), ciprofloxacin (5 µg), nalidixic acid (30 µg), trimethoprim/sulfamethoxazole (25 µg), and nitrofurantoin (300 µg). Multiple drug resistance isolates are those that have been found to be resistant to at least 3 different classes of antimicrobial drugs.
3.3. Extended-Spectrum β-Lactamases Screening Test
To screen extended-spectrum β-lactamases (ESBLs) producing isolates, the double-disk synergy test (DDST), was performed based on the CLSI guidelines. E. coli ATCC 25922 (negative control) and Klebsiella pneumoniae ATCC 700603 (positive control) were used as control strains based on the CLSI guidelines.
3.4. Detection of PAI Markers and Serogroups of UPEC Strains
Fresh colonies were used to extract bacterial DNA using the method previously reported (23). Using the exact primers given in Table 1, eight PAIs were detected (ie, PAI I536, PAI II536, PAI III536, PAI IV536, PAI ICFT073, PAI IICFT073, PAI IJ96, and PAI IIJ96). Initial denaturation at 94°C for 5 minutes was followed by 35 cycles of denaturation at 94°C for 1 minute, primer annealing at 55°C for 1 minute, and extension at 72°C for 1 minute with a final extension step at 72°C for 8 minutes (12). Moreover, with the specific primers provided in Table 1, PCR was performed to analyze O1, O2, O4, O6, O7, O8, O15, O16, O18, O21, O22, O25, O75, and O83 genes. Initial denaturation at 94°C for 5 minutes was followed by 30 cycles of primer annealing at 55°C for O1, 58°C for O2 and O25, and 56°C for O4 and O16 for 30 seconds, as well as an extension at 72°C for 30 seconds and a final extension at 72°C for 5 minutes. The KBC power load dye and 1.5% agarose gel were used to evaluate the amplification results (CinnaGen Co, Iran).
| Target | Primer Sequence (5′→3′) | Product Size (bp) |
|---|---|---|
| PAI I536-F | TAATGC CGG AGATTC ATT GTC | 1800 |
| PAI I536-R | AGG ATT TGT CTC AGG GCT TT | |
| PAI II536-F | CAT GTC CAA AGC TCG AGC C | 1000 |
| PAI II536-R | CTA CGT CAG GCT GGC TTT G | |
| PAI III536-F | CGG GCATGC ATC AAT TAT CTT TG | 200 |
| PAI III536-R | TGT GTA GAT GCA GTC ACT CCG | |
| PAI IV536-F | AAG GAT TCG CTG TTA CCG GAC | 300 |
| PAI IV536-R | TCG TCG GGC AGC GTT TCT TCT | |
| PAI ICFT073-F | GGA CAT CCT GTTACA GCG CGC A | 930 |
| PAI ICFT073-R | TCG CCA CCA ATC ACA GCG AAC | |
| PAI IICFT073-F | ATG GAT GTT GTATCG C | 400 |
| PAI IICFT073-R | ACG AGC ATG TGG ATC TGC | |
| PAI IJ96-F | TCG TGC TCA GGT CCG GAATTT | 400 |
| PAI IJ96-R | TGG CAT CCC ACATTATCG | |
| PAI IIJ96-F | GGATCC ATG AAA ACATGG TTA ATG GG | 2300 |
| PAI IIJ96-R | GAT ATT TTT GTT GCC ATT GGT TAC C | |
| O1 | GTGAGCAAAAGTGAAATAAGGAACG | 1098 |
| CGCTGATACGAATACCATCCTAC | ||
| O6 | GGATGACGATGTGATTTTGGCTAAC | 783 |
| TCTGGGTTTGCTGTGTATGAGGC | ||
| O7 | CTATCAAAATACCTCTGCTGGAATC | 610 |
| TGGCTTCGAGATTAAACCTATTCCT | ||
| O8 | CCAGAGGCATAATCAGAAATAACAG | 448 |
| GCAGAGTTAGTCAACAAAAGGTCAG | ||
| O16 | GGTTTCAATCTCACAGCAACTCAG | 302 |
| GTTAGAGGGATAATAGCCAAGCGG | ||
| O21 | CTGCTGATGTCGCTATTATTGCTG | 209 |
| TGAAAAAAAGGGAAACAGAAGAGCC | ||
| O75 | GAGATATACATGGGGAGGTAGGCT | 511 |
| ACCCGATAATCATATTCTTCCCAAC | ||
| O2 | AGTGAGTTACTTTTTAGCGATGGAC | 770 |
| AGTTTAGTATGCCCCTGACTTTGAA | ||
| O4 | TTGTTGCGATAATGTGCATGTTCC | 664 |
| AATAATTTGCTATACCCACACCCTC | ||
| O15 | TCTTGTTAGAGTCATTGGTGTATCG | 183 |
| ATAAAACGAGCAAGCACCACACC | ||
| O18 | GTTCGGTGGTTGGATTACAGTTAG | 551 |
| CTACTATCATCCTCACTGACCACG | ||
| O22 | TTCATTGTCGCCACTACTTTCCG | 468 |
| GAAACAGCCCATGACATTACTACG | ||
| O25 | AGAGATCCGTCTTTTATTTGTTCGC | 230 |
| GTTCTGGATACCTAACGCAATACCC | ||
| O83 | GTACACCAGGCAAACCTCGAAAG | 362 |
| TTCTGTAAGCTAATGAATAGGCACC |
Abbreviation: PAI, pathogenicity island.
3.5. Statistical Analysis
The data were analyzed using SPSS version 20 (SPSS Inc, Chicago, IL, USA). A chi-square or Fisher exact test was used to determine any statistical association. P values less than 0.05 were considered statistically significant.
4. Results
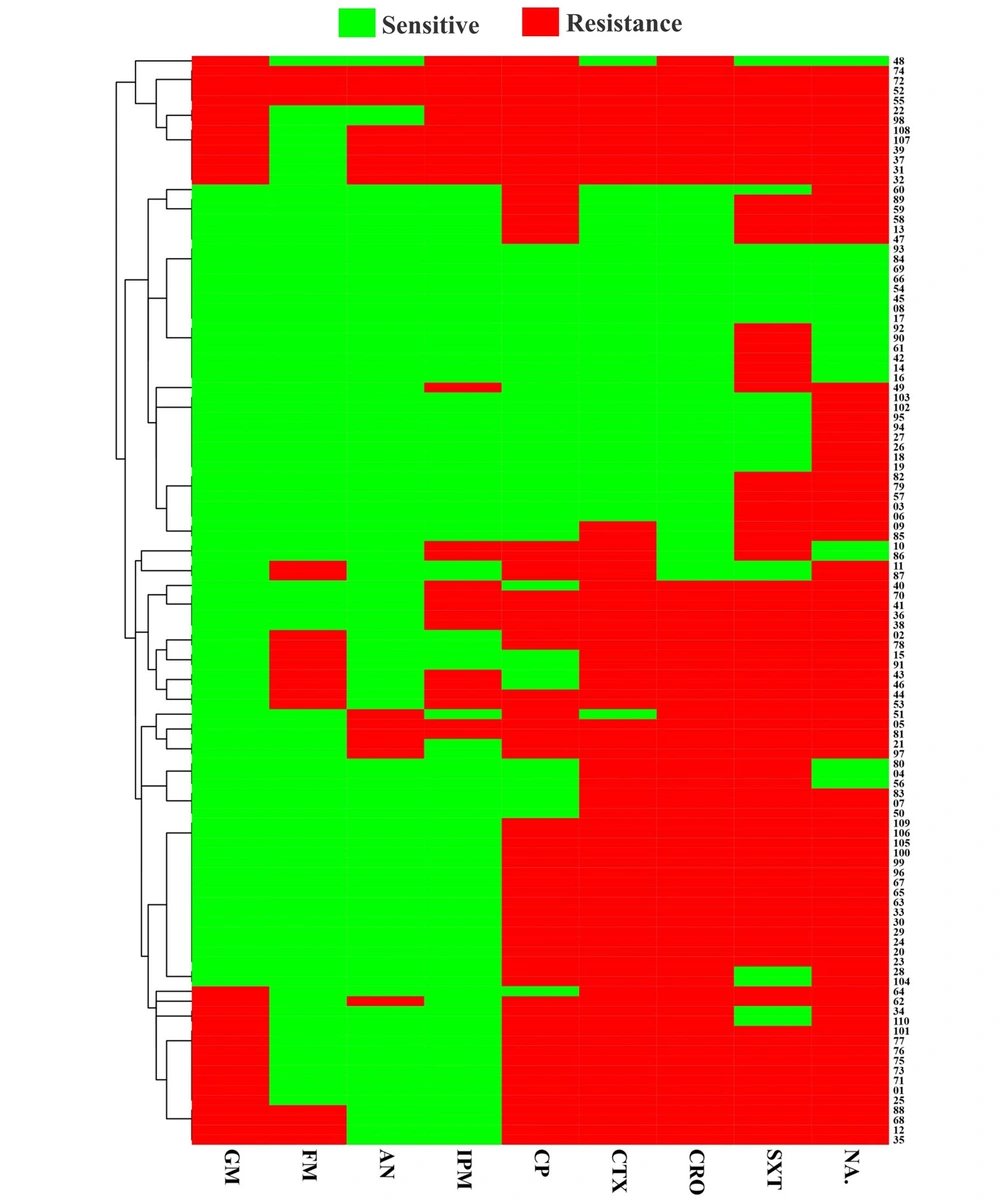
A total of 110 confirmed UPEC isolates were obtained from patients referred to a teaching hospital in Rasht, Iran. Among isolated samples, male and female frequencies were 36.7% (40/110) and 63.3% (70/110), respectively. According to the antibiotic susceptibility pattern, a high level of antibiotic resistance was observed against nalidixic acid (81.8%) and co-trimoxazole (78.2%), while the most operative agents were amikacin (85.5%) and nitrofurantoin (83.6%). The heatmap showed hierarchical clustering of all isolates’ antibiotic resistance profiles (Figure 1). The number of distinct classes of antimicrobials to which UPEC isolates were resistant varied among employed antimicrobial agents, according to the isolates’ detailed resistance profiles (Figure 1). Double-disk synergy test revealed that the incidence of ESBL-positive strains was 62.7% (69/110). Moreover, the MDR phenotype was found in 72.7% (80/110) of UPEC isolates.
Of the 110 UPEC isolates recovered from patients with UTI, 106 (96.4%) carried at least one of the investigated PAI markers. The predominant PAI among UPEC isolates was PAI IV536 (81.8%), followed by PAI ICFT073 (60%), PAI III536 (12.7%), PAI IIJ196 (9.1%), PAI II536 (8.2%), PAI I536 (6.4%), and PAI J196 (6.4%) (Table 1). According to our results, the most predominant serogroup O was O25 (36.4%), followed by O16 (17.3%), while the O4 and O7 serogroups (0.9%) were the lowest serogroups among UPEC isolates. Also, O22 and O83 were not detected in the studied strains (Table 2). The total spread of O-serogroups is summarized in Table 2. Compared to other serogroups, O25 and O16 serogroups showed the highest frequency of indicators for both antibiotic resistance and PAIs. All isolates from serogroup O25 possessed every single one of the examined PAI markers. Among the phylogenetic serogroups O8, O4, and O75, only a few virulence factor genes were more widely dispersed (Table 3). Furthermore, maximum resistance to ciprofloxacin, tetracycline, cefotaxime, and ceftazidime was observed in all UPEC serogroups, while minimum resistance to imipenem, amikacin, and nitrofurantoin was observed.
| PAI Markers | No. (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O21 | O18 | O16 | O15 | O8 | O7 | O6 | O4 | O2 | O75 | O1 | O25 | |
| PAI IIJ196 | 0 | 0 | 2 (10.5) | 1 (10) | 0 | 1 (100) | 1 (33.3) | 0 | 2 (50) | 0 | 0 | 2 (5) |
| PAI J196 | 1 (33.3) | 0 | 0 | 1 (10) | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 0 | 4 (10) |
| PAIIICFT073 | 1 (33.3) | 2 (66.7) | 5 (26.3) | 2 (20) | 0 | 0 | 2 (66.7) | 0 | 3 (75) | 1 (50) | 5 (55.6) | 13 (32.5) |
| PAI ICFT073 | 1 (33.3) | 2 (66.7) | 12 63.2) | 4 (40) | 2 (100) | 0 | 1 (33.3) | 1 (100) | 3 (75) | 1 (50) | 7 (77.8) | 25 (62.5) |
| PAI IV536 | 3 (100) | 2 (66.7) | 14 (7.37) | 7 (70) | 0 | 1 (100) | 1 (33.3) | 1 (100) | 4 (100) | 2 (100) | 8 (88.9) | 34 (85) |
| PAI III536 | 0 | 1 (33.3) | 3 (15.8) | 0 | 0 | 1 (100) | 0 | 0 | 2 (50) | 0 | 2 (22.2) | 2 (5) |
| PAI II536 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (100) | 0 | 0 | 0 | 0 | 0 | 5 (12.5) |
| PAI I536 | 0 | 0 | 1 (5.3) | 1 (10) | 0 | 1 (100) | 0 | 0 | 0 | 0 | 0 | 2 (5) |
| Total number of genes | 3 | 3 | 19 | 10 | 2 | 1 | 3 | 1 | 4 | 2 | 9 | 40 |
Abbreviation: PAI, pathogenicity island.
| Antibiotics | No. (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O21 | O18 | O16 | O15 | O8 | O7 | O6 | O4 | O2 | O75 | O1 | O25 | |
| Amikacin | 0 | 0 | 0 | 2 (20) | 1 (50) | 0 | 2 (66.7) | 0 | 3 (75) | 1 (50) | 1 (11.1) | 6 (15) |
| Imipenem | 1 (33.3) | 1 (33.3) | 1 (5.3) | 4 (40 | 1 (50) | 0 | 2 (66.7) | 0 | 3 (75) | 2 (100) | 2 (22.2) | 11 (27.5) |
| Nitrofurantoin | 0 | 1 (33.3) | 2 (10.5) | 3 (30) | 1 (50) | 0 | 2 (66.7) | 0 | 3 (75) | 2 (100) | 1 (11.1) | 4 (10) |
| Trimethoprim-sulfamethoxazole | 3 (100) | 3 (100) | 16 (84.2) | 8 (80) | 2 (100) | 0 | 1 (33.3) | 0 | 0 | 1 (50) | 7 (77.8) | 29 (72.5) |
| Cefotaxime | 3 (100) | 2 (66.7) | 15 (78.9) | 8 (80) | 2 (100) | 0 | 1 (33.3) | 0 | 1 (25) | 1 (50) | 6 (66.7) | 25 (62.5) |
| Gentamicin | 1 (33.3) | 0 | 7 (36.8) | 3 (30) | 1 (50) | 0 | 2 (66.7) | 0 | 3 (75) | 2 (100) | 1 (11.1) | 8 (20) |
| Ceftriaxone | 2 (66.7) | 2 (66.7) | 15 (78.9) | 8 (80) | 2 (100) | 0 | 2 (66.7) | 0 | 1 (25) | 1 (50) | 4 (44.4) | 24 (60) |
| Ciprofloxacin | 3 (100) | 2 (66.7) | 12 (63.2) | 6 (60) | 2 (100) | 0 | 2 (66.7) | 0 | 3 (75) | 1 (50) | 5 (55.6) | 23 (57.5) |
| Nalidixic acid | 2 (66.7) | 3 (100) | 18 (94.7) | 8 (80) | 2 (100) | 1 (100) | 1 (33.3) | 0 | 0 | 1 (50) | 5 (55.6) | 32 (80) |
| Total number of genes | 3 | 3 | 19 | 10 | 2 | 1 | 3 | 1 | 4 | 2 | 9 | 40 |
5. Discussion
Urinary tract infections are among the most common infections in both hospitals and communities worldwide. Escherichia coli strains that cause UTIs are thought to be the most prevalent bacteria in people of all sexes and ages (25). Nowadays, there are fewer options to treat infections, including UTIs, due to the dissemination of resistance genes and increased bacterial drug resistance in recent years. Furthermore, MDR E. coli infections are more difficult to cure in Asian nations like Iran (26)). In our investigation, 72.7% of the UPEC isolates were categorized as MDR, and 90% of the UPEC isolates were resistant to 1 or more antimicrobial drugs. The resistance to nalidixic acid and amikacin was found to be the highest and lowest, respectively, among all UPEC isolates in the current study. Previous studies from Pakistan (27), Iraq (28), and Iran (29) on UPEC isolates indicated a similar type of resistance to antibiotics. Considering what we have discovered, nitrofurantoin and amikacin appear to be effective antibiotics to treat UTIs caused by UPEC. Nasrollahian et al. showed that the highest frequency of MDR isolates was 76.3%, which is approximately in line with our results (5). In previous studies conducted in Spain (30), Iran (31), and Nepal (32), the percentages of MDR isolates were reported to be 30%, 55.8%, and 70.2%, respectively, which are much less compared to our results. Accordingly, the increase of MDR-Enterobacterales, in particular strains that produce ESBLs, is responsible for a high proportion of nosocomial outbreaks that are linked to higher morbidity and mortality rates (33, 34). In this regard, the hierarchical clustering of isolates’ antibiotic resistance profiles revealed a partial similarity in most UPEC isolates to the antibiotic-resistant pattern.
According to our results, 62.7% of isolates were ESBL producers. There have been numerous reports of ESBL-producing UPEC among hospitalized patients worldwide. According to a study in northern Iran, which is consistent with the current study, 66.3% of the UPEC isolates were ESBL-producing isolates (4). Moreover, previous studies in Kenya and China showed a lower frequency of ESBL-positive isolates (24.2% and 46%, respectively) (35, 36). A meta-analysis study estimated the proportion of ESBL-Enterobacteriaceae in East African hospitals, showing that the pooled average ESBL proportion for hospitals in East Africa was 42% (37). The types of hospital units, type of specimen, and site of infection are factors that influence variances in the prevalence of ESBL producers. Compared to patients who visit an outpatient department, hospitalized patients, particularly those in intensive care units, are often more prone to develop nosocomial infections that are likely to produce ESBLs (37).
Uropathogenic E. coli PAIs have encoded different virulence factors. These factors have a significant impact on the pathogenesis of UPEC strains and the development of the disease by impairing host defense mechanisms. The first PAIs were found in the UPEC genomes, and the PAIs of 120 different pathogen species have been discovered (5). Different virulence factors, such as particular adhesins, toxins, capsules, specialized O antigens, iron-uptake systems, and elements influencing serum resistance, can be produced by UPECs. The majority of these factors’ encoding genes are found in PAIs. In our investigation, among UPEC isolates, eight PAI markers were detected. Accordingly, 96.4% of UPEC isolates carried at least one of the investigated PAI markers. The highest and lowest PAIs were PAI IV536 (81.8%) and PAI J196 (6.4%) among UPEC isolates. This result is consistent with other investigations in Iran (38), China (24), the Czech Republic (39), and Sweden (40). Among UPEC strains known as high PAI, PAI IV536 has been described as the most common PAI.has been described as the most common PAI. The high frequency of PAI IV536 indicates that UPEC strains have steady levels in this marker. Moreover, 60% of UPEC isolates carried PAI ICFT073 as the second most common PAI. PAI ICFT073, known to include certain P fimbrial, toxin, and iron uptake system encoding genes, is effective to colonization and survival of E. coli strains in the human urinary tract (8).
In earlier research, PAIs of the 536 and CFT073 strains were linked to the highest number of PAI combinations. Early in the 1930s, systematic O-serogrouping of E. coli started, and it quickly became a crucial technique for identifying E. coli strains in clinical situations. Numerous experimental studies have found a strong correlation between specific serogroups and specific pathogenicity indicators in infections, such as UPEC. We found several O-serogroups in our UPEC isolates. Overall, O-serogroups O25, O16, O15, and O1 were the most frequently found, whereas the O4 and O7 serogroups were the least frequently found among the UPEC isolates in this study. Additionally, according to the findings of Momtaz et al., O25 (26.01%), O16 (10.56%), O4 (5.69%), O1 (2.43%), and O2 (2.4%) were the most frequently found serogroups among Iranian hospitalized patients (41).
According to Shokouhi Mostafavi et al., the 2 main O-serogroups among Iranian UPEC isolates were O1 (20%) and O25 (13.7%) (42). As reported by several researchers, in UTI patients, several O-serogroups were found to be present at different frequencies (41). According to the antibiotic susceptibility pattern, the isolates belonging to O25, O21, O16, O18, and O8 had the highest antibiotic resistance, while O4 and O7 had the lowest antibiotic resistance. Moreover, in the present research, the O25 serogroup had the highest distribution and incidence of PAI genes in UPEC, followed by O16, O18, and O7. Generally speaking, various O-serogroup distributions among UPEC isolates can fluctuate based on the type of infection, geography, or even other conditions (hospital or community) (14).
5.1. Conclusions
Based on the antibiogram, resistance to nalidixic acid and amikacin was found to be highest and lowest, respectively. Also, 62.7% of isolates were ESBL producers. In our investigation, the highest and lowest PAIs were PAI IV536 and PAI J196 among UPEC isolates. Moreover, O-serogroup O25 was the most frequently found, whereas the O4 and O7 serogroups were the least frequently found. The characterization of our strain revealed the co-occurrence of PAI and serogroups, confirming the importance of antibiotic resistance among the distinct serogroups and PAI markers. Our results have potential application for epidemiological studies and designing UTI treatment strategies against UTIs caused by UPEC.