1. Background
The finding of antibiotics is among the greatest human attainments of the 20th century. However, the effectiveness of any healing agent is limited by the possible evaluation of resistance (1). Globally, antibiotic resistance causes approximately seven hundred thousand deaths annually, which is expected to surpass 10 million by 2050 (2). For this reason, the World Health Organization identified pathogens for which antimicrobials are needed, and between the most serious priorities was extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-E) (3).
The increase in the incidence of community-acquired infections and ESBL-E infections is the reason for the increase in the frequency of approximately 50% in the last ten years (4). Most of these enzymes inactivate penicillin and cephalosporin and are common in Escherichia coli (5). This bacterium is the most commonly isolated microorganism in complicated and uncomplicated hospital or community-acquired urinary tract infections (UTI) (6). ESBL-E are often sensitive to carbapenems and cannot inactivate non-beta-lactam agents. However, microorganisms transporting ESBL genes often have genes or mutations that can confer resistance to other antibiotics, resulting in resistance to multiple antimicrobial groups and difficulty selecting treatment (7).
Local epidemiology and susceptibility results guide the selection of empiric antimicrobial therapy until the results of antimicrobial susceptibility tests are obtained (8). However, risk factors for resistant microorganisms should be evaluated in addition to the infection site and severity when selecting an antimicrobial agent (9). As the older population has more risk factors, UTI is a common cause of hospital admission in this group. Early Identifying risk factors and initiating appropriate antimicrobial therapy may improve clinical outcomes (10, 11).
2. Objectives
Therefore, in this study, we aimed to guide empiric antibiotherapy selection by determining the risk factors and resistance patterns in older outpatients ESBL-producing E. coli (ESBL-E. coli).
3. Methods
3.1. Patients
For this retrospective cohort study, we evaluated the data of 1066 patients aged 65 and older who presented to the outpatient clinics between 2011 and 2019 and had bacterial growth in their urine cultures. The study included all patients over 65 years who were admitted to the outpatient infectious diseases outpatient clinic between 2011 and 2019, whose urine cultures were positive for E. coli as the causative microorganism, and who were given antibiotic treatment with a diagnosis of UTI. A total of 815 patients (76.5%) who were diagnosed as having UTI and whose urine cultures were positive for E. coli were included in the study. The first sample was used for analysis if the same organism was isolated multiple times.
3.2. Isolates and Identification
Urine culture isolates with ≥ 105 colony-forming units (cfu)/mL concentrations after incubation at 37°C for 18 - 24 hours were accepted as pathogenic organisms. A Phoenix™ (Becton Dickinson Diagnostics, USA) automated microbiology system was used for the identification and antibiotic susceptibility testing of bacteria isolated from urine cultures. Antibiotic susceptibility results were evaluated according to Clinical Laboratory Standard Institute (CLSI) criteria. ESBL detection was performed using the same automated system. The patients’ clinical characteristics were obtained from their electronic records. We retrospectively evaluated the culture and antimicrobial susceptibility results of samples obtained from the patients. Antimicrobial agents were categorized as aminoglycosides (gentamicin and amikacin), antipseudomonal penicillins + beta-lactamase inhibitors (piperacillin-tazobactam), carbapenems (ertapenem, imipenem, meropenem), third- and fourth-generation cephalosporins (ceftriaxone, cefotaxime, ceftazidime), fluoroquinolones (ciprofloxacin), folate pathway inhibitors (trimethoprim-sulfamethoxazole), penicillins + beta-lactamase inhibitors (amoxicillin-clavulanic acid, ampicillin-sulbactam), phosphonic acid derivatives (fosfomycin), and nitrofurantoin (12).
3.3. Statistical Analysis
The data were analyzed using the IBM SPSS Statistics version 21.0 statistical software package. For descriptive statistics, categorical data were presented as frequency and percentage, and continuous variables were presented as median, minimum, and maximum values. Differences between the groups were compared for significance using the chi-squared (χ2) test for categorical variables and the Mann-Whitney U test or Fisher’s exact test for continuous variables. P-values < 0.05 was considered statistically significant.
4. Results
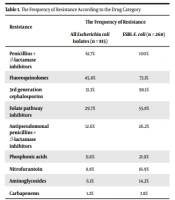
The patients added to the work had a median age of 73 years (range = 65 - 105), and 625 (58.6%) were female. The frequency of resistance according to drug category is indicated in Table 1. ESBL-E. coli was detected in 260 patients (31.9%). The highest resistance rates were to penicillin + β-lactamase inhibitors (61.7% overall, 100% among ESBL-E. coli). All resistance rates lower than 20% were observed for carbapenems, aminoglycosides, nitrofurantoin, fosfomycin, and antipseudomonal penicillins + β-lactamase inhibitors. ESBL-E. coli isolates indicated less than 20% resistance to carbapenems, nitrofurantoin, and aminoglycosides.
| Resistance | The Frequency of Resistance | |
|---|---|---|
| All Escherichia coli Isolates (n = 815) | ESBL-E. coli (n = 260) | |
| Penicillins + β-lactamase inhibitors | 61.7% | 100% |
| Fluoroquinolones | 45.8% | 73.1% |
| 3rd generation cephalosporins | 31.3% | 98.1% |
| Folate pathway inhibitors | 29.7% | 55.8% |
| Antipseudomonal penicillins + β-lactamase inhibitors | 12.6% | 26.2% |
| Phosphonic acids | 11.8% | 21.9% |
| Nitrofurantoin | 8.8% | 16.9% |
| Aminoglycosides | 6.1% | 14.2% |
| Carbapenems | 1.2% | 3.8% |
Abbreviation: ESBL-E. coli, extended-spectrum beta-lactamase-producing Escherichia coli
The comparison of risk factors according to ESBL positivity in patients with E. coli in urine culture is presented in Table 2. Risk factors for ESBL-producing bacteria were determined. These were the presence of benign prostatic hypertrophy, antibiotic use in the last three months, history of UTI in the last year, urinary catheter uses in the last year, male gender, and hospitalization in the last year (P < 0.05). Analysis to determine risk factors for ESBL-E. coli included male sex, benign prostatic hypertrophy, urinary catheter use in the last year, UTI in the last year, antibiotic use in the last three months, and hospitalization in the last year. The only independent risk factor was a history of UTI in the last year, which increased the risk of ESBL by 2.8 times. (95% confidence interval 1.509 - 5.119, P = 0.001).
| Factors | ESBL | P | |
|---|---|---|---|
| Positive (n = 260) | Negative (n = 555) | ||
| Age | 73 (65 - 95) | 72 (65 - 105) | 0.546 |
| Gender (male) | 128 (49.2) | 158 (28.6) | < 0.001 |
| Hypertension | 83 (31.9) | 196 (35.3) | 0.341 |
| Diabetes mellitus | 44 (16.9) | 116 (20.9) | 0.183 |
| Chronic obstructive pulmonary disease | 46 (17.7) | 100 (18.0) | 0.910 |
| Coronary artery disease | 56 (21.5) | 110 (19.8) | 0.570 |
| Chronic kidney disease | 8 (3.1) | 12 (2.2) | 0.431 |
| Nephrolithiasis | 9 (3.5) | 6 (1.1) | 0.019 |
| Benign prostatic hypertrophy | 57 (21.9) | 73 (13.2) | 0.001 |
| History of urological intervention in the last year | 31 (11.9) | 73 (13.2) | 0.624 |
| Antibiotic use in the last three months | 82 (31.5) | 129 (23.2) | 0.012 |
| Urinary tract infection in the last year | 64 (24.6) | 63 (11.4) | < 0.001 |
| Use of urinary catheter in the last year | 71 (27.3) | 72 (13.0) | < 0.001 |
| Hospitalization in the last year | 52 (20.0) | 59 (10.6) | < 0.001 |
Abbreviation: ESBL, extended-spectrum beta-lactamase
a Values are expressed as median (min - max) or No. (%).
5. Discussion
Urinary tract infection is a widespread diagnosis among older outpatients (10, 11, 13). As observed in this study, E. coli is the most common microorganism (55% - 95%) in both hospital- and community-acquired UTIs (6, 14). The increase in ESBL-producing microorganisms is a public health problem (3, 15-17). Knowing the risk factors for UTI caused by ESBL-producing microorganisms and the associated antimicrobial resistance patterns can facilitate patient management (8, 15). Therefore, our aim in this study was to determine the risk factors and resistance patterns of ESBL-E. coli UTI in older outpatients. Community-acquired UTI is significantly associated with the female gender over the age of 26 (18). In women with aging, decreased estrogen levels cause pH changes in the vagina, resulting in an increased risk of E. coli colonization and UTI. In men, prostate enlargement causes urinary obstruction, increasing the risk of residual urine and infection. Decreased bladder capacity, voiding volume with aging, and urinary storage and evacuation problems are other physiological changes that increase the risk of UTI (19, 20). With these physiological changes, the frequency of urinary tract infections increases in both sexes, especially over 65 years (20).
Previous history is one of the strongest predictors of future UTI in older adults (21). Besides sexually active young women, recurrent UTI is also common in the older population, attributed to insufficient bladder emptying, personal hygiene problems, increased comorbidity, and frequent urinary catheterization (22). In studies reported from our country, the frequency of ESBL-producing organisms in community-acquired UTIs among all age groups was between 7.4% and 38.2% (6, 14, 23, 24). This rate was high (31.9%) in our study focusing on older adults, consistent with previous studies (25, 26).
In a case-control study in Italy, age, history of hospitalization, Charlson comorbidity index ≥ 4, recent use of beta-lactam antibiotic and/or fluoroquinolones, and recent urinary catheterization were identified as independent risk factors for community-acquired ESBL-E infections. In retrospective studies conducted in different age groups, recurrent UTI was also found to be an independent risk factor for community-acquired UTI caused by ESBL-producing microorganisms (15, 16, 27). Similarly, we determined that a history of UTI in the last year was an independent risk factor for ESBL-E. coli-associated UTI in the older adults in our study. This result supports the proposition that multidrug-resistant microorganisms emerged after the transfer of resistance genes and the widespread use of antibiotics (28).
Resistance genes are transferred via integrons, plasmids, and transposons. Plasmids encoding ESBL enzymes also carry genetic material against many antibiotics. ESBL genes have also been shown in integron-like structures. In our study, the increased frequency of resistance to other antibiotic groups in the presence of ESBL reflects this situation. This will lead to difficulties in the choice of antibiotic for treatment (29). In a systematic review and meta-analysis study evaluating class 1 integrons-associated antibiotic resistance in uropathogenic E. coli, the highest antibiotic resistance rate was observed in ampicillin (85%), and the lowest in imipenem (5%) and amikacin (12%). These results are consistent with our results (28). Inappropriate empirical antibiotic use impacts recurrent UTI, ultimately on bacterial ecology and the spread of antibiotic resistance (11).
Knowledge of antimicrobial resistance rates is important in determining appropriate empirical treatment. Therefore, conducting national, regional, and even hospital studies and also empiric agents with no more than 10 - 20% resistance are recommended (6). The frequency of resistance to fluoroquinolones, penicillins + beta-lactamase inhibitors, and third-generation cephalosporins was high in our study, as previously reported (14, 23). Therefore, caution should be exercised in the empiric use of these agents. In the guidance on resistant antimicrobial therapy, nitrofurantoin and trimethoprim-sulfamethoxazole are recommended as first-line treatment in ESBL-E cystitis, with single-dose aminoglycosides and fosfomycin recommended as alternatives (7). However, as in our study, reports from our country have demonstrated high rates of resistance to trimethoprim-sulfamethoxazole and stated that this drug should be investigated as a therapeutic option (14, 24). A similarly high resistance level to trimethoprim-sulfamethoxazole has been observed in community-acquired uropathogenic ESBL-E. coli reported from a nearby region outside our country (29).
We observed a relatively lower frequency of resistance to nitrofurantoin, fosfomycin, and aminoglycosides, consistent with the literature (6, 14). On the other hand, it is recommended to avoid nitrofurantoin and oral fosfomycin for upper UTIs because they do not reach sufficient concentrations in the renal parenchyma. In such cases, carbapenems and fluoroquinolones are reported to be preferable as first-line therapies (7). In our study, the antimicrobials with the lowest resistance rates were carbapenems. However, we observed over 50% resistance to ciprofloxacin in ESBL-E. coli, consistent with previous studies in our country (14, 24, 30). The strength of our research is the large sample of older adults included. However, a limitation of this retrospective study was our inability to define the duration and doses of antibiotic therapy and urinary catheter duration in all patients.
5.1. Conclusions
Monitoring antimicrobial resistance is important to guide appropriate empiric antimicrobial therapy. ESBL-producing microorganisms should be considered first in older patients with recurrent UTI. Nitrofurantoin and fosfomycin can be considered first-line antibiotic therapy. But fluoroquinolones and trimethoprim-sulfamethoxazole should also be avoided.