1. Background
Bacillus clausii is known as a probiotic bacterium. Previous research has shown that B. clausii has anti-bacterial properties, but the current data are insufficient. Recent studies have reported that the antimicrobials produced by B. clausii have activities against Clostridium difficile and Staphylococcus aureus in vitro (1). Bacillus clausii produces the class I antibiotic clausin, which works against Gram-positive bacteria (2). According to the current research, B. clausii has no anti-bacterial action on Gram-negative germs but has an inhibitory effect on some Gram-positive germs. More research is required to determine the range of its antimicrobial properties. Several bacilli have shown antiviral action against human viruses, such as B. subtilis to inhibit the HSV and influenza virus (3), B. horneckiae against herpes simplex virus 1 (4), and B. licheniformis against herpes simplex virus 2 (5). Additionally, the ribonuclease (binase) of B. pumilus has been developed as an inhibitor of rhinovirus serotype 1A and influenza A in cell culture (6). The direct activity of B. clausii and its metabolites against human viruses is unknown and needs more focus.
Probiotics have been shown to have anticancer properties. Also, B. licheniformis, B. subtilis, B. coagulans, and B. cereus have been studied for their ability to suppress cancer development or trigger apoptosis (7). However, there is little knowledge of B. clausii. Bacillus strains have been assessed for their effects on the BAX and BCL-2 genes in cancer cells (8-10), but no data have been published on B. clausii and cervical cancer HeLa cells. In addition, the possible role of Bacilli has not been assessed in upregulating the expression level of miR-145 involved in apoptosis.
2. Objectives
This work aimed to get further information on the anti-bacterial activity of B. clausii supernatant. Additionally, the possible inhibitory effect of B. clausii supernatant against adenovirus type 5 (Ad5) is investigated. Adenoviruses are one of the most common causes of respiratory diseases in humans. However, no study has published data on the inhibitory effect of probiotics on adenoviruses. We also aimed to evaluate the impact of the bacterial supernatant on the expression of genes associated with apoptosis in the HeLa cell line, such as BAX, BCL-2, and miR-145.
3. Methods
3.1. Bacteria, Cell Line, and Virus
Bacillus clausii (ATCC 700160) was purchased as lyophilized from the Iranian Biological Resource Center (IBRC). Fifty milliliters of B. clausii culture (106 CFU/mL) was cultured in a tryptic soy broth medium for 24 h. The culture suspensions were centrifuged (700 g, 25 minutes) to extract the cell-free supernatants. The IBRC provided S. aureus (ATCC 25923), Escherichia coli (ATCC 25922), Acinetobacter baumannii (ATCC 1960), Methicillin-resistant S. aureus (MRSA) (ATCC 43300), Pseudomonas aeruginosa (ATCC 27853), and Enterococcus faecalis (ATCC 25912). The virology department at Tarbiat Modares University (Tehran, Iran) provided HeLa cells, HEK-293 cells, and Ad5 as gifts.
3.2. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
Anti-bacterial activity of B. clausii was assessed on S. aureus, MRSA, E. faecalis, E. coli, and P. aeruginosa. Several colonies of isolates were put into the Brain Heart Infusion (BHI) medium (HiMedia, India) and cultivated for 24 h at 37°C. One hundred microliters of bacterial suspensions (1.5 × 105 CFU/mL) were added into 96-well plates and treated with 100 µL of different concentrations of each compound (dilutions 1/2 to 1/28). After 24 h storing at 37°C, the Minimum Inhibitory Concentration (MIC) was determined as the lowest dose at which the bacteria could not grow visibly. To find the minimum bactericidal concentration (MBC), TSB tubes with the lowest B. clausii supernatant were used to inoculate Trypticase soy agar using the pour plate technique (11).
3.3. Adenovirus Titration
According to a previously described procedure, we carried out a median tissue culture infectious dose (TCID50) experiment to evaluate the titer of adenovirus type 5 (12).
3.4. Cytotoxicity Assay of Bacillus clausii Supernatant
The HeLa cells were grown in cell culture microplates (20,000 cells per well) in triplicate to test the cytotoxicity of B. clausii supernatant. Dimethylthiazolyl-diphenyl tetrazolium bromide (MTT) test was used to determine cytotoxicity using a previously described procedure (13).
3.5. Antiviral Activity Assay
We cultured HEK-293 cells in six-well plates. Bacterial supernatants (dilutions 1/4 and 1/8) and Ad5 (MOI = 0.1) were added to cells in different conditions, including pre-treatment, pre-incubation, competition, and post-treatment, as previously described (14). The Ad5-infected cells were considered a control (without exposure to the bacterial supernatant).
3.6. Relative Expression of E1A Adenovirus and BAX, BCL-2, and microRNA-145 Genes in HeLa Cancer Cells
The E1A expression levels in different experimental assays were measured to approve TCID50 results. A real-time PCR test determined the level of the BCL-2, BAX, and microRNA-145 transcripts to evaluate the influence of B. clausii supernatant on the apoptosis activation in the HeLa cell line. First, 1 × 105 HeLa cells were grown in each well of six-well plates. The cells were treated with dilutions of bacterial supernatant (1/4 and 1/8) for 24 h and 48 h at 37°C. The RNX kit (SinaClon, Iran) isolated total RNA from the cultivated cells. A commercial cDNA Synthesis Kit (AddBio, South Korea) was used to transcribe one microgram of total RNA.
The cDNA for the miR-145 and U6 genes (as a control gene) was synthesized using the following RT primers: RT-U6–5′-ATATGGAACGCTTCACGAATTTGC-3′, RT-miR-145-5p-5′-TCGTATCCAGTGCAGGGTC CGAGGTATTCGCACTGGATACGACAG. The real-time PCR was done in the ABI StepOnePlusTM equipment and specific primers (Table 1). The PCR mixture included 12.5 µL of Ampliqon master mix (Denmark), 0.5 µL (10 µmol) of each primer, 2 µL of cDNA, and 9.5 µL of Distilled Water (DW). Amplifications were carried out using the following cycling profile: 94°C for 10 minutes and then 40 cycles, including 20 s at 95°C, 30 s at 60°C, and 30 s at 72°C. The results were normalized against the expression of human beta-globin (U6 for miR-145), and the 2-ddCT technique was utilized to estimate the relative expression of genes.
| Primers | Sequence (5-3) | Product Size (bp) | References |
|---|---|---|---|
| miR145-forward | TCCAGTTTTCCCAGGAATCCC | 85 | (12) |
| miR145-reverse | ATCCAGTGCAGGGTCCGA | ||
| U6-forward | GAGAAGATTAGCATGGCCCCT | 90 | (12) |
| U6-reverse | ATATGGAACGCTTCACGAATTTGC | ||
| Bax-forward | CCTGTGCACCAAGGTGCCGGAACT | 99 | (15) |
| Bax-reverse | CCACCCTGGTCTTGGATCCAGCCC | ||
| Bcl-2-forward | TTGTGGCCTTCTTTGAGTTCGGTG | 114 | (15) |
| Bcl-2-reverse | GGTGCCGGTTCAGGTACTCAGTCA | ||
| B-Actin-forward | GGCGGCACCACCATGTACCCT | 202 | (15) |
| B-Actin-reverse | AGGGGCCGGACTCGTCATACT | ||
| E1A-forward | ACCTCCTAGCCATTTTGAACCAC | 122 | (12) |
| E1A-reverse | GTCAATCCCTTCCTGCACCG |
3.7. Statistical Analysis
GraphPad Prism 6.0 software was used to compare TCID50 and real-time PCR data between the control and treated groups by a student's t-test.
4. Results
4.1. Minimum Inhibitory Concentration/Minimum Bactericidal Concentration
By determining the MIC and MBC, the anti-bacterial activity of B. clausii supernatant was examined against several bacterial genera (Table 2). The cell-free supernatant of B. calusii had an inhibitory effect on E. faecalis, S. aureus, MRSA, E. coli, P. aeruginosa, and A. baumannii. The anti-bacterial effect of E. faecalis occurred at a lower concentration of B. calusii supernatant than that of other bacteria. The bacterial supernatant had no bactericidal activity against MRSA, P. aeruginosa, and A. baumannii.
| Test | Enterococcus faecalis | Staphylococcus aureus | MRSA | Escherichia coli | Pseudomonas aeruginosa | Acinetobacter baumannii |
|---|---|---|---|---|---|---|
| MIC | 1/32 | 1/16 | 1/4 | 1/16 | 1/4 | 1/4 |
| MBC | 1/16 | 1/8 | - | 1/8 | - | - |
4.2. Cell Viability Assay
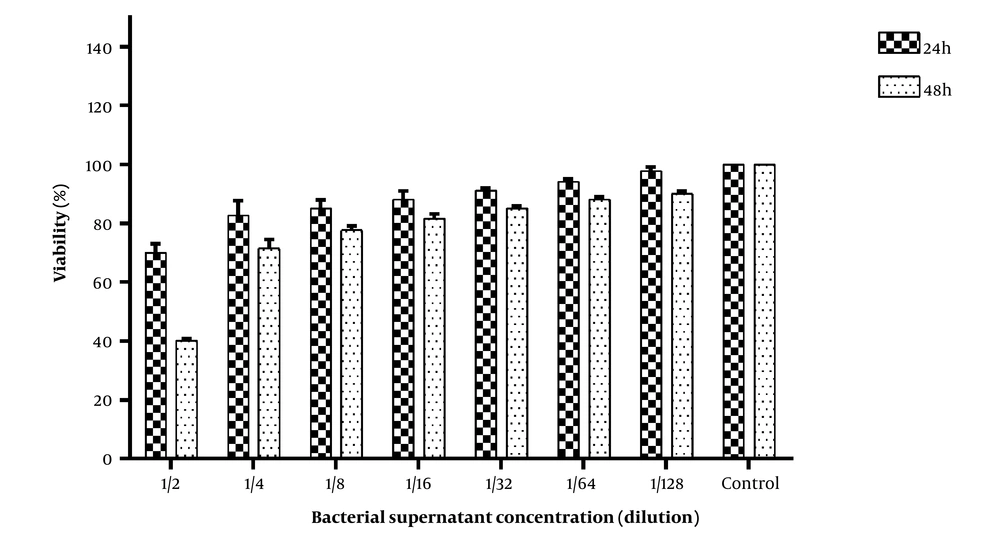
The results of HeLa cell viability are shown in Figure 1. The MTT findings revealed that incubating HeLa cells for 24 and 48 hours with bacterial supernatant at a concentration of 1/2 reduced viable cells proportion to 65% and 40%, respectively. Additionally, 80% of cells were still alive after exposure to CFS at concentrations above 1/4 for 24 h and above 1/8 for 48 h.
4.3. Anti-adenoviral Effect of Bacillus clausii Supernatant
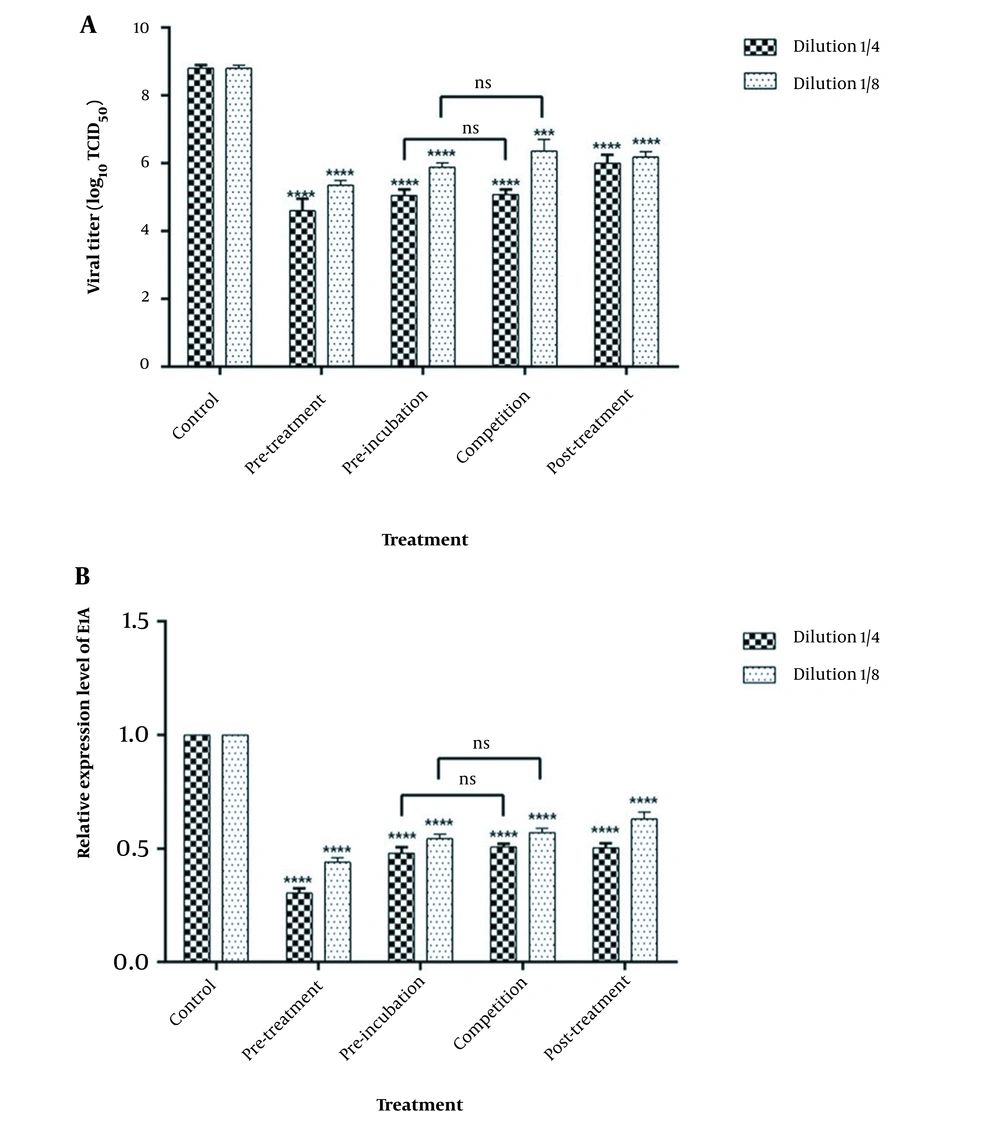
The human Ad5 titer was 8.91 ± 0.14 Log10 TCID50/mL when B. clausii supernatant was absent (control). The Ad5 titer was calculated after incubation of CFS (dilution: 1/4 and 1/8) and HEK-293 cells in different conditions. Data are summarized in Table 3 and Figure 2. The Ad5 titer in pre-treatment, pre-incubation, competition, and post-treatment conditions with a concentration of 1/4 supernatant dropped by 4.61, 4, 3.9, and 3.1 Log10 TCID50/mL, respectively. As demonstrated in Figure 2, the Ad5 titer was significantly lower (P-value 0.05) in all experiments than in the control group. The mean Ad5 titer in the pre-incubation and competition tests was similar. Similar results of the viral titration were shown when the E1A expression in the experimental and control tests was compared (Figure 2B). The most significant reduction in E1A-adenovirus expression was seen in the pre-treatment assay.
| Different Experimental Assays | Mean Ad5 Titer (Log10 TCID50/mL ± SD) | |
|---|---|---|
| Supernatant Dilution: 1/4 | Supernatant Dilution: 1/8 | |
| Pre-treatment | 4.30 ± 0.28 | 5.33 ± 0.14 |
| Pre-incubation | 4.91 ± 0.16 | 5.91 ± 0.28 |
| Competition | 5.00 ± 0.14 | 6.08 ± 0.16 |
| Post-treatment | 5.75 ± 0.16 | 6.33 ± 0.28 |
| Control | 8.91 ± 0.14 | 8.91 ± 0.14 |
Human Ad5 titer (A) and E1A expression level (B) in different experimental conditions of Bacillus clausii inoculation. Statistical differences between the mean virus titer (or E1A expression) at each stage of bacterial inoculation and the mean virus titer in the absence of bacteria (control) were analyzed by the student test (ns: Not significant; **** P-value < 0.0001, *** P-value < 0.001).
4.4. Effect of Bacillus clausii Supernatant on the Expression of BAX, BCL-2, and miR-145 Genes
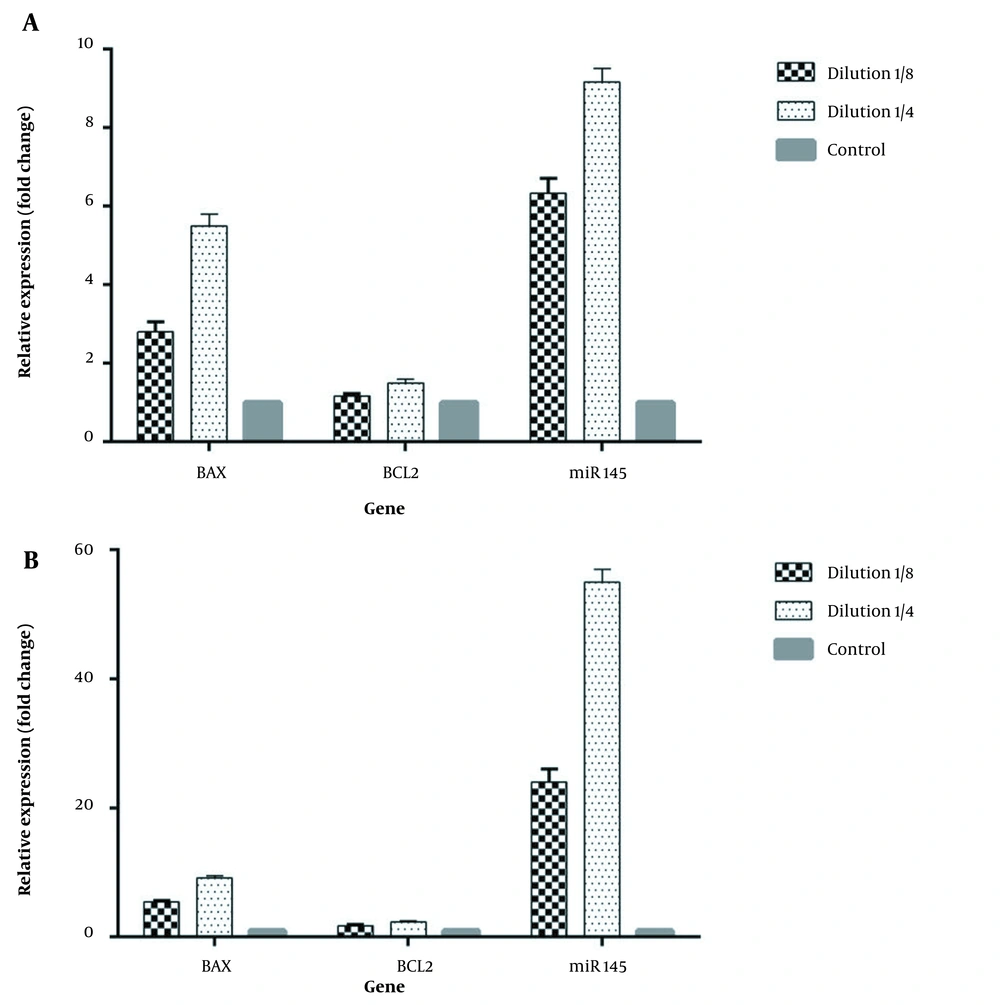
When B. clausii supernatant was added to HeLa cells for 24 h at a concentration of 1/8, the levels of BAX, BCL-2, and miR-145 genes increased to 2.8, 1.16, and 6.3 folds, respectively, compared with untreated cells. In addition, 1/4 dilution of the bacterial supernatant during 48 h increased BAX, BCL-2, and miR-145 genes by 5.5, 1.5, and 9.1 folds (Figure 3). A concentration of 1/8 B. clausii supernatant during 48 h could elevate the level of BAX, BCL-2, and miR-145 genes by 5.4, 1.7, and 24 folds, respectively, compared to the untreated condition.
5. Discussion
Several Bacillus species are investigated in humans as potential probiotic candidates. The current study evaluated the anti-bacterial, anti-adenoviral, and apoptotic effects of B. clausii supernatant. Data showed that the B. clausii supernatant inhibits some bacterial strains and human adenovirus type 5. Furthermore, it remarkably induced the level of the apoptosis-linked genes BAX and miR-145. Several studies have demonstrated that B. clausii strains produce metabolites with bacterial inhibitory and anti-fungal effects. In the current work, B. clausii supernatant after 24 h of culture had anti-bacterial effects on E. faecalis, S. aureus, MRSA, E. coli, P. aeruginosa, and A. baumannii. Clausin is a lantibiotic recently isolated from B. clausii and is active against some gram-positive microbes (3). In the previous studies, it inhibited M. luteus at MIC = 16 mg/L and MRSA at MIC = 128 mg/L (4). Similarly, in the present study, B. clausii had an inhibitory effect on MRSA at a MIC equal to 1/4 dilution of the bacterial supernatant. Ripert et al. showed that compounds secreted by B. clausii inhibit toxins of two pathogens, C. difficile and B. cereus (16). Using a colony overlay test, Ripert et al. examined the inhibitory effect of the strains O/C, N/R, SIN, and T of B. clausii, which are probiotics.
The B. clausii CFS had inhibitory effects on S. aureus, E. faecium, Lactobacillus lactis, and C. difficile but had no inhibitory effect on gram-negative germs (E. coli, Salmonella typhimurium, S. flexneri, Vibrio cholerae, V. parahaemolyticus, and P. fluorescens) (1). In the present work, contrary to the findings of Urdaci et al., B. clausii supernatant had an antimicrobial effect on E. coli, P. aeruginosa, and A. baumannii. This disagreement in results may be due to variations in the research techniques or strains utilized in the two investigations. One of the aims of the present study was the in vitro assessment of the anti-adenoviral activity of B. clausii. The B. clausii CFS showed an antiviral impact on all tests conducted in different conditions. However, the pre-treatment condition showed the highest drop in Ad5 titer. The virus's titer was 4.61 Log10 TCID50/mL lower than the control. It is conceivable that the supernatant of B. clausii can cause interference in adenovirus adhesion to the target cell. As a result, it keeps the virus from entering the cell. This finding was obtained in a few investigations on other probiotics. The culture supernatant of vaginal lactobacilli displayed a strong neutralizing effect on herpes simplex virus type 2 before viral entrance (17). Contrary to the present study, Mousavi et al. found that the CFS of L. crispatus did not show a significant antiviral activity on HSV (18).
MicroRNA-145 is a tumor suppressor often expressed in healthy, normal cells but drastically downregulated in malignant cells (12, 19). Recent research has suggested that miR-145 may be essential for controlling tumor cell growth, migration, invasion, and death in many malignancies (20). According to Pan et al., raising miR-145 levels in A549 cells resulted in a notable rise in the expression of BAX and a decline in the expression of MMP2, MMP9, and BCL-2. The A549 cells' apoptosis is accelerated by increasing the proportion of BAX to BCL-2 (21). The molecular mechanisms by which miR-145 promotes apoptosis in HeLa cells are unknown. However, Li et al. suggested that miR-145 can increase cell death in the HeLa cell line by modulating Wnt/β-catenin (20). In the current study, 24 and 48-hour incubations of B. clausii supernatant with HeLa cell line increased the level of miR-145 by 24 and 55 folds, respectively. Therefore, B. clausii supernatant can have an anti-tumor effect on HeLa cells through increasing tumor suppressor miR-145. This finding has been reported in a few previous studies. Fahmy et al. found that B. longum can induce the production of microRNA-145 in murine colorectal tumors (22). In the study of Anton et al., the expression of miRNA145 in cervical cells was upregulated after contact with bacteria-free supernatants of Gardnella vaginalis (23).
5.1. Conclusions
Bacillus clausii can be a potent antimicrobial and anticancer agent. However, further studies can determine its anti-bacterial, antiviral, and anticancer activity spectrum.