1. Background
Acinetobacter baumannii is a Gram-negative, nonfermentative, obligate aerobic, coccobacilli that have an ubiquitous distribution in nature being recovered from soil or water, human skin, and respiratory tract (1, 2). Acinetobacter baumannii is an important nosocomial pathogen, with a rising prevalence of about 89.2% among hospitalized patients, especially in ICU patients (3). Antimicrobial treatment of these infections may be compromised by the multiple-drug resistance of many strains to β-lactams, aminoglycosides, and fluoroquinolones (4). This bacterium causes a variety of diseases including urinary tract infections, endocarditis, surgical site infections, pneumonia, septicemia, and meningitis (1, 5). Numerous strains of A. baumannii have recently emerged to be resistant against a wide spectrum of antibiotics, particularly β-lactams, which have so far been among drug choices for treatment of A. baumannii infections. Consequently, treatment of these infections has encountered serious problems (2, 6).
Antibiotic resistance genes, including β-Lactamase genes, are the main molecular basis of microbial drug resistance; and plasmids along with the mobile genetic elements such as transposons and integrons are behind the rapid distribution of these multiple resistance genes (7). According to the Amber classification, β-Lactamases are categorized in 4 classes, A-D, based on their amino acid sequence similarities (8). Almost all beta-lactamases including the classic ESBLs, namely TEM, SHV, and CTX-M types, as well as non-classic ESBLs such as VEB and PER types belong to the class A Amber β-lactamases, with the exception of OXA type classic ESBLs like OXA-2 and OXA-10, which categorized in the group D of Amber classification (9, 10). Classic ESBLs have all originated from the parent types in TEM (temoneria), SHV (sulfhydryl variable), and OXA (oxacillinase) families. However, there are newer families of ESBLs discovered during the last 2 - 3 decades, such as VEB (for Vietnamese Extended-spectrum Beta-lactamase) and PER (for Pseudomonas extended resistance) families, among them, VEB-1 and PER-1 being the major types (11). Five different classes of integrons have been identified and INT-1 is the most prevalent type in clinical isolates of gram negative bacilli, including A. baumanii (12, 13).
Determination of antimicrobial resistance patterns and genotypic background of the ESBL production in clinical isolates of MDR bacteria would shed light on their epidemiology and contribute to the wise adoption of proper preventative or therapeutic measures (14). Unfortunately, there is a lack of well documented data on Acinetobacter antimicrobial resistance pattern and gene prevalence in Iran.
2. Objectives
The aim of this study was to determine the antibacterial resistance pattern and molecular prevalence of blaTEM, blaSHV, blaCTX-M, blaOXA-2 and blaOXA-10 genes among the clinical isolates of A. baumanii from patients hospitalized in the Imam Reza hospital of Tabriz, North-west Iran.
3. Methods
3.1. Sampling, Bacterial Isolation and Identification
The study was approved by the ethical committee of Tabriz University of Medical Sciences (code: 76/488). A. baumannii isolates were recovered from various clinical specimens of hospitalized patients in different wards of the Imam Reza hospital of Tabriz as follow: ICU, neurosurgery, internal wards, infectious wards, general surgery unit, and ENT. The clinical specimens were collected from invasive and non-invasive sites including tracheal aspirate, urine, sputum, blood, bronchial washing, catheter, wound, abscess drainage, cerebrospinal fluid (CSF), pleural effusion, and ascitic fluid. The samples were immediately incubated in MacConkey and blood agar (Merck, US) media (37°C for 24 - 48 minutes). The initial identification of isolates was carried out using the standard microbiological and biochemical methods, including Gram-staining, colony morphology, glucose oxidation, citrate utilization, oxidase test, O/F (Oxidation-Fermentation) test, catalase test, and growth ability at 44°C (15, 16).
3.2. Antibiotic Susceptibility Testing
To determine the antibiotic susceptibility pattern of isolates, Kirby Bauer disk diffusion testing was carried out using the clinical laboratory standard institute (CLSI) guidelines (17). Antibiotic disks used were ceftriaxone (30 µg), ceftazidime (30 µg), cefepime (30 µg), tetracycline (30 µg), rifampin (5 µg), gentamicin (10 µg), cephalexin (30 µg), amikacin (30 µg), ciprofloxacin (5 µg), tobramycin(10 µg), levofloxacin (5 µg), ampicillin-sulbactam (10/10 µg), piperacillin (100 µg), piperacillin-tazobactam (100/10 µg), ticarcillin-clavulanic acid (75/10 µg), imipenem (10 µg) meropenem (10 µg), colistin (10 µg), and polymyxin B (300 units) (MAST, UK). The disks were placed on Mueller-Hinton agar (Merck, Germany) plates and inoculated with bacterial suspension equal to 0.5 McFarland at 37°C overnight. The diameter of the zone of growth inhibition was measured using the CLSI guidelines.
3.3. Double-Disk Synergy Test
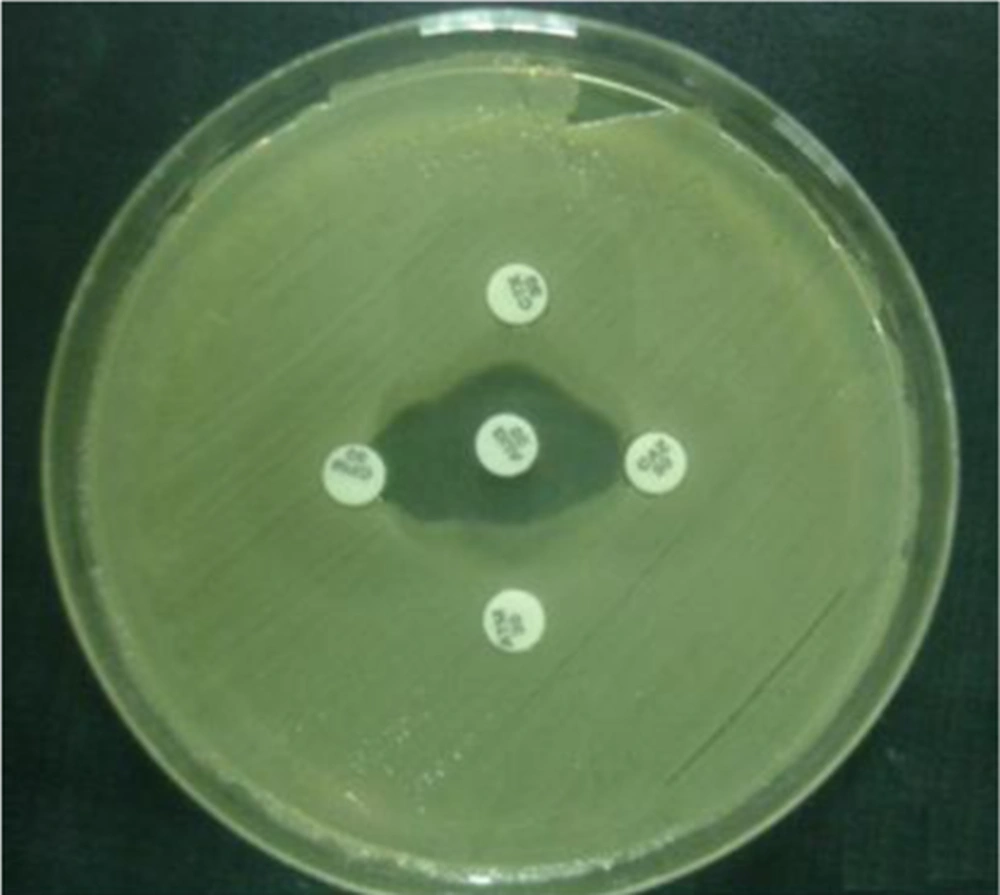
The double-disk synergy test was used to identify extended-spectrum β-lactamase (ESBL)-producing strains. The antibiotic disks of amoxicillin-clavulanic acid (20 μg/10 μg), cefotaxime (30 μg), ceftazidime (30 μg), and aztreonam (30 μg), obtained from Nissui Pharmaceuticals were used for the test. Amoxicillin-clavulanic acid disk was placed on the core of the plate and the 4 other disks mentioned above were placed in a 10 mm distance from the central disk and 20 mm from each other (18) (Figure 1).
3.4. DNA Extraction and PCR Amplification
DNA was extracted from the isolates using the SDS-Proteinase K and phenol-chloroform method (19). After an overnight culture, fresh colonies was resuspended in TE buffer containing SDS (1%) and proteinase K (10 μg/mL) (Fermentas, Lithuania), and incubated for 3 hours at 40°C, followed by extraction with phenol-chloroform and ethanol precipitation. Finally, the quantity and quality of extracted DNA was checked via spectroscopy and agarose gel electrophoresis, respectively.
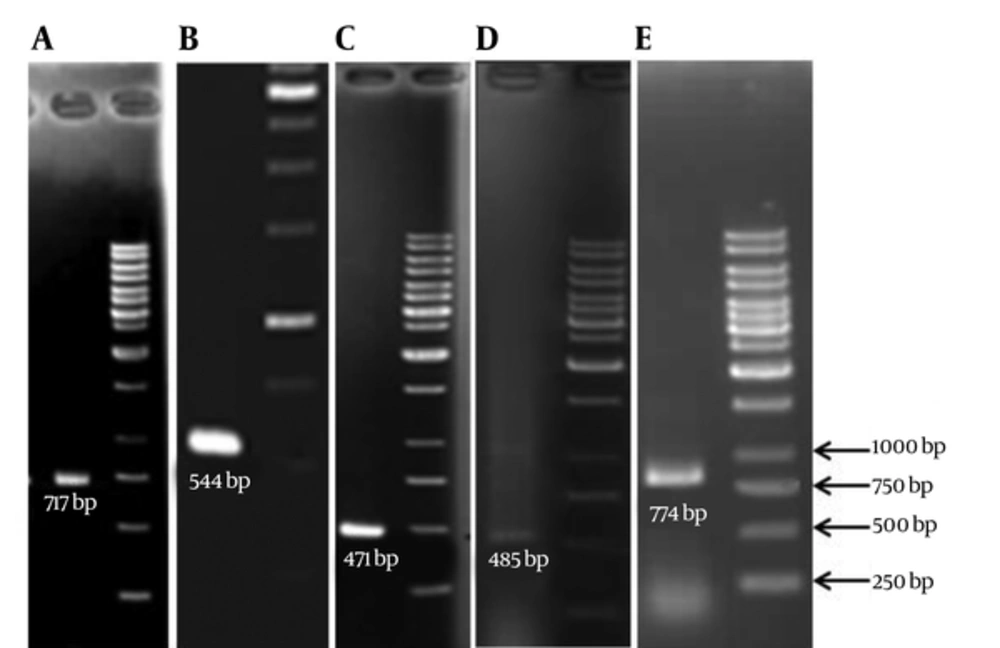
The PCR technique was used to detect the presence of ESBL genes (TEM, SHV, CTX-M, OXA2, and OXA10) in the clinical isolates of A. baumannii using specific primers (Table 1). PCR for blaOXA-51-like genes was also carried out to confirm the identity of isolates (20). PCR products were analyzed by agarose gel electrophoresis in TAE buffer. The gel was then stained with ethidium bromide for 20 minutes and visualized by gel documentation system.
| Primer | Primer Sequences | Annealing Temp, °C | Product Size, bp |
|---|---|---|---|
| TEM-F | 5'-CTTCCTGTTTTTGCTCACCCA-3' | 68 | 717 |
| TEM-R | 5'-TACGATACGGGAGGGCTTAC-3' | ||
| SHV-F | 5'-TCAGCGAAAAACACCTTG-3' | 60 | 471 |
| SHV-R | 5'-TCCCGCAGATAAATCACC-3' | ||
| CTX-M-Fa | 5'- SCSATGTGCAGYACCAGTAA-3' | 60 | 544 |
| CTX-M-R | 5'- CCGCRATATGRTTGGTGGTG-3' | ||
| OXA2-F | 5'- AAGAAACGCTACTCGCCTGC -3' | 60 | 485 |
| OXA2-R | 5'- CCACTCAACCCATCCTACCC -3' | ||
| OXA10-F | 5'-TATCGCGTGTCTTTCGAGTA-3' | 55 | 774 |
| OXA10-F | 5'-TTAGCCACCAATGATGCC-3' | ||
| OXA51-F | 5'-ACAAGCGCTATTTTTATTTCAG-3' | 51 | 641 |
| OXA51-R | 5'-CCCATCCCCAACCACTTTT-3' |
The Sequence of Primers Used for PCR Amplification of Resistance Genes
4. Results
4.1. Patients and Bacterial Isolation
A total of 100 A. baumannii isolates were recovered from various clinical specimens including trachea (37%), urine (21%), sputum (9%), blood (7%), catheter (6%), bronchial wash (5%), wound (3%, abscess (3%), and other (6%). The age range of the patients was from 14 to 86 years, where 25% of isolates belonged to patients between the age of 20 - 39 years and 72% were from patients between 40 - 90 years old.
4.2. Bacterial Identification
The results of the biochemical test demonstrated the identity of all the isolates as A. baumannii species. Further PCR analyses showed presence of blaOXA-51-like genes in isolates, which confirmed their identity as A. baumannii.
4.3. Antimicrobial Susceptibility
Results of antimicrobial susceptibility testing showed that the highest antibiotic resistance rate were against ticarcillin (100%), cefixime (100%), and ceftizoxime (100%), followed by aztreonam (97%), cephalexin (97%), cefotaxime (97%), ampicillin (94%), kanamycin (94%), ceftriaxone (94%), ceftazidime (93%), cephalothin (91%), carbenicillin (89%), co-trimoxazole (85%), norfloxacin (84%), ciprofloxacin (80%), chloramphenicol (78%), gentamicin (78%), ofloxacin (71%), tetracycline (65%), tobramycin (63%), meropenem (63%), imipenem (62%), ampicillin-sulbacta (55%) whereas the highest susceptibility rates were noticed for polymyxin B (84%), colistin (77%), and rifampin (73%).
4.4. Genotypic Characterization of ESBL-Positive Isolates
Double-Disk Synergy Test showed that 60% of the isolates were ESBL producers. PCR experiments (Figure 2), for detection of ESBL genotypes of TEM, SHV, CTX-M, OXA-2 and OXA-10 among the ESBL positive isolates revealed that the highest frequency belonged to the SHV (31.6%) whereas the lowest frequency were for OXA10 (8.3%) (Table 2).
| Gene | No. (%) | Gene Coincidence | No. (%) |
|---|---|---|---|
| TEM | 7 (11.6) | TEM + SHV + CTX-M + PER1 + INT-1 | 1 (1.6) |
| SHV | 19 (31.6) | SHV + CTX + INT-1 | 2 (3.3) |
| CTX-M | 8 (13.3) | PER1 + VEB1 + INT-1 | 3 (5) |
| OXA2 | 7 (11.6) | TEM + CTX-M | 3 (5) |
| OXA10 | 5 (8.3) | TEM + SHV | 4 (6.6) |
| OXA2 + OXA10 + INT-1 | 3 (5) | ||
| OXA2 + INT-1 | 7 (11.6) | ||
| OXA10 + INT-1 | 5 (8.3) |
Prevalence of ESBL Genotypes in the ESBL-Positive Clinical Isolates (N = 60)
Among 60 ESBL producing isolates, 1 (1.66%) were positive for TEM, SHV, and CTX-M genes, 2 (3.33%) had both CTX-M and SHV, 3 (5%) possessed both CTX-M and TEM genes, and finally 4 isolates (6.66%) contained both SHV and TEM genes together.
5. Discussion
Recent reports have indicated that the antimicrobial resistance rates of Acinetobacter isolates are continuously increasing, consequently posing a growing threat to hospitalized patients (21, 22). Nosocamial strains of Acinetobacter use different antibiotic resistance mechanisms such as reduced access to target caused by alterations in cell wall channels (porins), increased antibiotic expulsion by efflux pumps, enzymatic inactivation by β-lactamases, mutational resistance, and biofilm formation (23). It has been shown that enzymatic degradation by β-lactamases is the most common mechanism in A. baumannii (2).
In this study the results of antimicrobial susceptibility testing showed that all studied strains were resistant against ticarcillin, cefixime, and ceftizoxim (100% resistance); while, 84% of them were susceptible for polymyxin B, 77% for colistin, and 73% for rifampin, indicating the highest susceptibility rates. Similar A. baumanii susceptibility rates had been reported in previous studies in Iran. Shahcheraghi et al. (24) determined resistance rates of 100% against cefixime, 99% against ceftriaxone, and 98% against cefotaxime, along with susceptibility rates of 97% for polymyxin B and 88% for colistin in A. baumanii isolates from Tehran. Mohajeri et al. (25) reported the highest resistance rates against cefotaxime (93%) and ceftriaxone (91%), whilst the highest susceptibility rates were found to colistin (89%) and polymyxin B (86%) in A. baumanii isolates from Kermanshah. These results suggest that polymyxin B and colistin may be helpful in treating A. baumanii-related nosocomial infections in hospital settings.
Findings of this study showed that 60% of the isolates were ESBL-positive, indicating a high frequency of ESBL-associated resistance, whereas reports from India and Turkey indicated a frequency of 28% and 39%, respectively (26, 27). Genotypic screening revealed that 11.6%, 13.3%, and 31.6% of isolates were positive for blaTEM, blaCTX-M, and blaSHV, respectively. By contrast, a study from China reported that the frequency of blaTEM, and blaCTXM positive isolates were 25% and 66%, respectively (28). Furthermore, a study in South America reported that the frequencies of blaTEM, blaCTX-M, and blaSHV genotypes among the studied Acinetobacter spp. were 26.1%, 30.4%, and 8.7%, respectively (29). Nevertheless, our study shows a higher frequency of blaSHV and lower frequencies of blaTEM and blaCTX-M, compared to the mentioned studies.
In our study the prevalence of blaOXA-2 and blaOXA-10 genes were 11.6% and 8.3%, respectively. OXA-type β-Lactamases are characterized by their hydrolysis potency on cloxacillin and oxacillin, which are 50% greater than that for benzylpenicillin (30). Of the 244 known OXA-type β-Lactamases, only 16 of them are known as ESBLs, among which OXA-2 and OXA-10 considered as parent types for the rest (31). Most acquired OXA-type β-lactamases including OXA-2, OXA-10, and their derivatives are associated with class 1 integron or other insertion sequences (32). Integrons play an important role in distributors of acquired drug resistance genes among bacteria and A. baumanii (13). Accordingly, our previous study showed a high prevalence of INT-1 insertion sequence (75%) among the ESBL producing strains (33). Different frequency of INT-1 gene in A. baumanii isolates had been reported in other countries including 27.53% from Spain (8) and 71.4% from Taiwan (13), where the last prevalence rate was similar to our results.
In conclusion, our results revealed a high level of antimicrobial resistance among the studied clinical isolates of A. baumanii, and demonstrated the vital role of the studied ESBL genes in the generation of this antimicrobial resistance. These findings emphasize on the necessity of antimicrobial surveillance in different geographic regions for control of resistance dissemination. Such studies are critical to take proper measures in respect of prevention, patient care or therapy, in order to control further propagation of these severely resistant bacterial strains in community and hospital settings.